Abstract
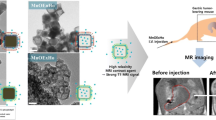
Iron oxide nanoparticles (IONPs) with high-index facets have shown great potential as high performance T2 contrast agents for MRI. Previous synthetic approaches focused mainly on ion-directed or oxidative etching methods. Herein, we report a new synthetic route for preparing high-index faceted iron oxide concave nanocubes using a bulky coordinating solvent. Through the systematic replacement of a non-coordinating solvent, 1-octadecene, with trioctylamine, the solvent interaction with the nanoparticle surface is modified, thereby, promoting the growth evolution of the IONPs from spherical to concave cubic morphology. The presence of the bulky trioctylamine solvent results in particle size increase and the formation of nanoparticles with enhanced shape anisotropy. A well-defined concave nanocube structure was evident from the early stages of particle growth, further confirming the important role of bulky coordinating solvents in nanoparticle structural development. The unique concave nanocube morphology has a direct influence on the magnetic properties of the IONPs, ultimately leading to an ultra-high T2 relaxivity (862.2 mM−1 s−1), and a 2-fold enhancement in T2*-weighted in vivo MRI contrast compared to spherical IONP analogs.






Similar content being viewed by others
References
Lee N, Choi Y, Lee Y, Park M, Moon WK, Choi SH, Hyeon T. Water-dispersible ferrimagnetic iron oxide nanocubes with extremely high r2 relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett. 2012;12:3127–31.
Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem Soc Rev. 2012;41:2575–89.
Todd T, Zhen Z, Tang W, Chen H, Wang G, Chuang YJ, Deaton K, Pan Z, Xie J. Iron oxide nanoparticle encapsulated diatoms for magnetic delivery of small molecules to tumors. Nanoscale. 2014;6:2073–6.
Huang J, Wang L, Zhong X, Li Y, Yang L, Mao H. Facile non-hydrothermal synthesis of oligosaccharide coated sub-5 nm magnetic iron oxide nanoparticles with dual MRI contrast enhancement effects. J Mater Chem B. 2014;2:5344–51.
Yoo D, Lee JH, Shin TH, Cheon J. Theranostic magnetic nanoparticles. Acc Chem Res. 2011;44:863–74.
Zhao Z, Zhou Z, Bao J, Wang Z, Hu J, Chi X, Ni K, Wang R, Chen X, Chen Z, Gao J. Octapod Iron oxide nanoparticles as high performance T2 contrast agents for magnetic resonance imaging. Nat Commun. 2013;4:2266.
Smolensky ED, Park HYE, Zhou Y, Rolla GA, Marjańska M, Botta M, Pierre VC. Scaling laws at the nanosize: the effect of particle size and shape on the magnetism and relaxivity of iron oxide nanoparticle contrast agents. J Mater Chem B. 2013;1:2818–28.
Roch A, Muller RN, Gillis P. Theory of proton relaxation induced by superparamagnetic particles. J Chem Phys. 1999;110:5403–11.
Zhou Z, Zhu X, Wu D, Chen Q, Huang D, Sun C, Xin J, Ni K, Gao J. Anisotropic shaped iron oxide nanostructures: controlled synthesis and proton relaxation shortening effects. Chem Mater. 2015;27:3505–15.
Situ SF, Samia ACS. Highly efficient antibacterial iron oxide@carbon nanochains from wüstite precursor nanoparticles. ACS Appl Mater Interfaces. 2014;6:20154–63.
Redl FX, Black CT, Papaefthymiou GC, Sandstrom RL, Yin M, Zeng H, Murray CB, O’Brien SP. Magnetic, electronic, and structural characterization of nonstoichiometric iron oxides at the nanoscale. J Am Chem Soc. 2004;126:14583–99.
Park J, An KJ, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater. 2004;3:891–5.
Jun YW, Choi JS, Cheon J. Shape control of semiconductor and metal oxide nanocrystals through nonhydrolytic colloidal routes. Angew Chem Int Ed. 2006;45:3414–39.
Mohanty A, Garg N, Jin R. A universal approach to the synthesis of noble metal nanodendrites and their catalytic properties. Angew Chem Int Ed. 2010;49:4962–6.
Zeng H, Rice PM, Wang SX, Sun S. Shape-controlled synthesis and shape-induced texture of MnFe2O4 nanoparticles. J Am Chem Soc. 2004;126:11458–9.
Liang H, Jiang X, Qi Z, Chen W, Wu Z, Xu B, Wang Z, Mi J, Li Q. Hematite concave nanocubes and their superior catalytic activity for low temperature CO oxidation. Nanoscale . 2014;6:7199–203.
Hofmann C, Rusakova I, Ould-Ely T, Prieto-Centurión D, Hartman KB, Kelly AT, Lüttge A, Whitmire KH. Shape control of new FexO–Fe3O4 and Fe1–yMnyO–Fe3–zMnzO4 nanostructures. Adv Funct Mater. 2008;18:1661–7.
Bao L, Low WL, Jiang J, Ying JY. Colloidal synthesis of magnetic nanorods with tunable aspect ratios. J Mater Chem. 2012;22:7117–20.
Jin M, Zhang H, Xie Z, Xia Y. Palladium concave nanocubes with high-index facets and their enhanced catalytic properties. Angew Chem Int Ed. 2011;50:7850–4.
Shao Z, Zhu W, Wang H, Yang Q, Yang S, Liu X, Wang G. Controllable synthesis of concave nanocubes, right bipyramids, and 5-fold twinned nanorods of palladium and their enhanced electrocatalytic performance. J Phys Chem C. 2013;117:14289–94.
Lee PY, Teng HS, Yeh CS. Preparation of superparamagnetic MnxFe1-xO nanoparticles from low-index-facet cubes to high-index-facet concave structures and their catalytic performance in aqueous solution. Nanoscale. 2013;5:7558–63.
Kovalenko MV, Bodnarchuk MI, Lechner RT, Hesser G, Schäffler F, Heiss W. Fatty acid salts as stabilizers in size- and shape-controlled nanocrystal synthesis: the case of inverse spinel iron oxide. J Am Chem Soc. 2007;129:6352–3.
Macher T, Totenhagen J, Sherwood J, Qin Y, Gurler D, Bolding MS, Bao Y. Ultrathin iron oxide nanowhiskers as positive contrast agents for magnetic resonance imaging. Adv Funct Mater. 2015;25:490–4.
Cozzoli PD, Snoeck E, Garcia MA, Giannini C, Guagliardi A, Cervellino A, Gozzo F, Hernando A, Achterhold K, Ciobanu N, Parak FG, Cingolani R, Manna L. Colloidal synthesis and characterization of tetrapod-shaped magnetic nanocrystals. Nano Lett. 2006;6:1966–72.
Li Z, Ma Y, Qi L. Controlled synthesis of MnxFe1−xO concave nanocubes and highly branched cubic mesocrystals. CrystEngComm . 2014;16:600–8.
Palchoudhury S, Xu Y, Rushdi A, Holler RA, Bao Y. Controlled synthesis of iron oxide nanoplates and nanoflowers. Chem Commun. 2012;48:10499–501.
Douglas FJ, MacLaren DA, Tuna F, Holmes WM, Berry CC, Murrie M. Formation of octapod MnO nanoparticles with enhanced magnetic properties through kinetically controlled thermal decomposition of polynuclear manganese complexes. Nanoscale. 2014;6:172–6.
Liao HG, Zherebetskyy D, Xin H, Czarnik C, Ercius P, Elmlund H, Pan M, Wang LW, Zheng H. Facet development during platinum nanocube growth. Science. 2014;345:916–9.
Bronstein LM, Huang X, Retrum J, Schmucker A, Pink M, Stein BD, Dragnea B. Influence of iron oleate complex structure on iron oxide nanoparticle formation. Chem Mater. 2007;19:3624–32.
Ding X, Bao L, Jiang J, Gu H. Colloidal synthesis of ultrathin γ-Fe2O3 nanoplates. RSC Adv. 2014;4:9314–20.
Samia AC, Schlueter JA, Jiang JS, Bader SD, Qin CJ, Lin XM. Effect of ligand− metal interactions on the growth of transition-metal and alloy nanoparticles. Chem Mater. 2006;18:5203–12.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds, Part B: Applications in coordination, organometallic, and bioinorganic chemistry. 2nd edn. New York: Wiley; 2009.
Lu Y, Miller JD. Carboxyl stretching vibrations of spontaneously adsorbed and LB-transferred calcium carboxylates as determined by FTIR internal reflection spectroscopy. J Colloid Interface Sci. 2002;256:41–52.
Tandon P, Förster G, Neubert R, Wartewig S. Phase transitions in oleic acid as studied by X-ray diffraction and FT-Raman spectroscopy. J Mol Struct. 2000;524:201–15.
Hufschmid R, Hamed A, Ferguson RM, Gonzales M, Teeman E, Brush LN, Browning ND, Krishnan KM. Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale. 2015;7:11142–54.
Wang YXJ. Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant Imaging Med Surg. 2011;1:35–40.
Yu WW, Xiaogang P. Formation of high‐quality CdS and other II–VI semiconductor nanocrystals in noncoordinating solvents: tunable reactivity of monomers. Angew Chem Int Ed. 2002;41:2368–71.
Huang X, Zhao Z, Fan J, Tan Y, Zheng N. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J Am Chem Soc. 2011;133:4718–21.
Qiao L, Fu Z, Li J, Ghosen J, Zeng M, Stebbins J, Prasad PN, Swihart MT. Standardizing size-and shape-controlled synthesis of monodisperse magnetite (Fe3O4) nanocrystals by identifying and exploiting effects of organic impurities. ACS Nano. 2017;11:6370–81.
Acknowledgements
This work was supported by a NSF-CAREER Grant (DMR-1253358) from the Solid State and Materials Chemistry Program. Tilting TEM and SAED analyses on the concave nanocubes were performed with assistance from SCSAM at CWRU. The HRTEM data was obtained at the TEM facility at the Liquid Crystal Institute, Kent State University, supported by the Ohio Research Scholars Program Research Cluster on Surfaces in Advanced Materials. The authors thank the technical support of Dr. Min Gao for the HRTEM measurement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Situ-Loewenstein, S.F., Wickramasinghe, S., Abenojar, E.C. et al. A novel synthetic route for high-index faceted iron oxide concave nanocubes with high T2 relaxivity for in vivo MRI applications. J Mater Sci: Mater Med 29, 58 (2018). https://doi.org/10.1007/s10856-018-6052-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6052-6