Abstract
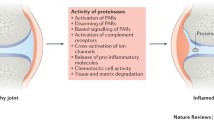
Plant extracts with a high content of proteolytic enzymes have been used in traditional medicine for a long time. Besides herbal proteinases, ‘modern’ enzyme therapy includes pancreatic enzymes. The therapeutic use of proteolytic enzymes is empirically based, but is also supported by scientific studies. This review provides an overview of preclinical and clinical trials of systemic enzyme therapy in rheumatic disorders. Studies of the use of proteolytic enzymes in rheumatic disorders have mostly been carried out on enzyme preparations consisting of combinations of bromelain, papain, trypsin and chymotrypsin. The precise mechanism of action of systemic enzyme therapy remains unresolved. The ratio of proteinases to antiproteinases, which is affected by rheumatic diseases, appears to be influenced by the oral administration of proteolytic enzymes, probably via induction of the synthesis of antiproteinases or a signal transduction of the proteinase-antiproteinase complex via specific receptors. Furthermore, there are numerous alterations of cytokine composition during therapy with orally administered enzymes resulting from immunomodulatory effects, which might be an indication of the efficacy of enzyme therapy.
The results of various studies (placebo-controlled and comparisons with nonsteroidal anti-inflammatory drugs) in patients with rheumatic diseases suggest that oral therapy with proteolytic enzymes produces certain analgesic and anti-inflammatory effects. However, the results are often inconsistent. Nevertheless, in the light of preclinical and experimental data as well as therapeutic experience, the application of enzyme therapy seems plausible in carefully chosen patients with rheumatic disorders.



Similar content being viewed by others
Notes
The use of trade names is for identification purposes only, and does not imply endorsement.
References
Vanhoof G, Cooreman W. Bromelain. In: Lauwers A, Scharpé S, editors. Pharmaceutical enzymes. New York: Marcel Dekker, 1997: 131–53
De Feo V. Medicinal and magical plants in the northern Peruvian Andes. Fitoterapia 1992; 53: 417–40
Rawlings ND, Barrett AJ. Evolutionary families of peptidases. Biochem J 1993; 290: 205–18
Rowan AD, Buttle DJ, Barrett AJ. The cysteine proteinases of the pineapple plant. Biochem J 1990; 266: 869–75
Harrach T, Eckert K, Schulze-Forster K, et al. Isolation and partial characterization of basic proteinases from stem bromelain. J Protein Chem 1995; 14: 41–52
Christie RB. The medical uses of proteolytic enzymes. In: Wiseman A, editor. Topics in enzyme and fermentation biotechnology. Vol. 4. Chichester: Ellis Horwood, 1980: 25–83
Netti C, Bandi GL, Pecile A. Anti-inflammatory action of proteolytic enzymes of animal vegetable or bacterial origin administered orally compared with that of known anti-phlogistic compounds. Farmaco-Edizione Pratica 1972; 27: 453–66
Ito C, Yamaguchi K, Shibutani Y, et al. Anti-inflammatory actions of proteases, bromelain, trypsin and their mixed preparation. Nippon Yakurigaku Zasshi 1979; 75: 227–37
Wood GR, Ziska T, Morgenstern E, et al. Sequential effects of an oral enzyme combination with rutosid in different in vitro and in vivo models of inflammation. Int J Immunother 1997; 13: 139–46
Hashimoto Y, Kakegawa H, Narita Y, et al. Significance of cathepsin B accumulation in synovial fluid of rheumatoid arthritis. Biochem Biophys Res Commun 2001; 283: 334–9
Yoshihara Y, Nakamura H, Obata K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 2000; 59: 455–61
Borth W, Dunky A, Kleesiek K. α2-Macroglobulin-proteinase complexes as correlated with α1-proteinase inhibitor-elastase complexes in synovial fluids of rheumatoid arthritis patients. Arthritis Rheum 1986; 29: 319–25
Abbink JJ, Kamp AM, Nieuwenhuys EJ, et al. Predominant role of neutrophils in the inactivation of α2-macroglobulin in arthritic joints. Arthritis Rheum 1991; 34: 1139–50
Kruze K, Fehr K, Boni A. Effect of antirheumatic drugs on cathepsin B1 from bovine spleen. Z Rheumatol 1976; 35: 95–102
Keyszer G, Lambiri I, Keysser M, et al. Matrix metalloproteinases, but not cathepsin B, H, and L or their inhibitors in peripheral blood of patients with rheumatoid arthritis are potentially useful markers of disease activity. Z Rheumatol 1998; 57: 392–8
Fisher JD, Weeks RL, Curry WM, et al. Effects of an oral enzyme preparation, Chymoral®, upon serum proteins associated with injury (acute phase reactants) in man. J Med 1974; 5: 258–73
Latha B, Ramakrishnan KM, Jayaraman V, et al. Action of trypsin: chymotrypsin (Chymoral forte DS) preparation on acute-phase proteins following burn injury in humans. Burns 1997; 23: 3–7
Targoni OS, Tary-Lehmann M, Lehmann PV. Prevention of murine EAE by oral hydrolytic enzyme treatment. J Autoimmun 1999; 12: 191–8
Simon AK, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci U S A 1994; 91: 8562–6
Mazurov VI, Lila AM, Klimko NN, et al. The efficacy of systemic enzyme therapy in the treatment of rheumatoid arthritis. Int J Immunother 1997; 13: 85–92
Lehmann PV, Forsthuber T, Miller A, et al. Spreading of T-cell autoimmunity of cryptic determinants of an autoantigen. Nature 1992; 358: 155–7
LaMarre J, Wollenberg GK, Gonias SL, et al. Biology of disease. Cytokine binding and clearance properties of proteinase-activated α2-macroglobulins. Lab Invest 1991; 65: 3–13
Emancipator SN, Chintalacharuvu SR, Urankar Nagy N, et al. Effects of oral enzymes in collagen II induced arthritis in mice. Int J Immunother 1997; 13: 67–74
Kullich W, Schwann H. Circulating immune complexes and complement fragment iC3b in chronic polyarthritis during 12 months therapy with oral enzymes in comparison with oral gold. Wien Med Wochenschr 1992; 142: 493–7
Steffen C, Smolen J, Miehlke K, et al. Enzyme therapy in comparison with immune complex determinations in chronic polyarthritis. Z Rheumatol 1985; 44: 51–6
Singer F, Oberleitner H. Drug therapy of activated arthrosis. On the effectiveness of an enzyme mixture versus diclofenac. Wien Med Wochenschr 1996; 146: 55–8
Klein G, Kullich W. Short-term treatment of painful osteoarthritis of the knee with oral enzymes: a randomised, double-blind study versus diclofenac. Clin Drug Invest 2000; 19: 15–23
Klein G, Kullich W, Brugger A. Phlogenzym in der Behandlung der Periarthropathia humeroscapularis tendopathica simplex. Arzt Praxis 1997; 51: 879–85
Tilscher H, Keusch R, Neumann K. Results of a double-blind, randomized comparative study of Wobenzym-placebo in patients with cervical syndrome. Wien Med Wochenschr 1996; 146: 91–5
Wittenborg A, Bock PR, Hanisch J, et al. Therapie mit nichtsteroidalen Antiphlogistika versus einem oralen Enzymkombinationspräparat. Arzneimittelforschung 2000; 50: 728–38
Klein G. Study No. MU-692424: monocentric, randomized, double-blind study of efficacy and safety of Phlogenzym® in patients with periarthropathia humero-scapularis. MUCOS Pharma GmbH & Co, Geretsried, Germany 1994. Data on file
Klein G. Study No. MU-91407: Phlogenzym® in painful arthrosis. MUCOS Pharma GmbH & Co, Geretsried, Germany 1996. Data on file
Klein G. Study No. MU-693422: Phlogenzym® in the treatment of osteoarthritis of the knee joint. MUCOS Pharma GmbH & Co, Geretsried, Germany 1996. Data on file
Klein G. Study No. MU-91409: Phlogenzym® in the treatment of thoracic spine/lumbar spine symptoms. MUCOS Pharma GmbH & Co, Geretsried, Germany 1996. Data on file
Baumüller M. Study No. MU-696413: Phlogenzym® in patients with thoracic spine/lumbar spine syndrome. MUCOS Pharma GmbH & Co, Geretsried, Germany 1996. Data on file
Klein G. Study No. MU-695419: Phlogenzym® in the treatment of periarthritis humeroscapularis tendopathica. MUCOS Pharma GmbH & Co, Geretsried, Germany 1996. Data on file
Singer F. Study No. MU-695414: Phlogenzym® in the treatment of a monoarticular gonarthritis. MUCOS Pharma GmbH & Co, Geretsried, Germany 1997. Data on file
Herrera EG. Study No. MU-696416: Phlogenzym® in the treatment of a monoarticular painful gonarthritis. MUCOS Pharma GmbH & Co, Geretsried, Germany 1998. Data on file
Pavelka K. Study No. MU-693405: efficacy and safety of an oral hydrolytic enzyme therapy inpatients with rheumatoid arthritis. MUCOS Pharma GmbH & Co, Geretsried, Germany 2000. Data on file
Klein G. Study No. MU-696401: Phlogenzym® in the treatment of a monoarticular gonarthritis. MUCOS Pharma GmbH & Co, Geretsried, Germany 1997. Data on file
Klein G. Study No. MU-86215: efficacy and tolerance of Wobenzym as base therapy in rheumatoidal arthritis in comparison with oral gold. MUCOS Pharma GmbH & Co, Geretsried, Germany 1987. Data on file
Singer F. Study No. MU-87208: base therapy of rheumatoidal arthritis with Wobenzym® in comparison with oral gold therapy. Efficacy and tolerance in a randomised, open, parallel group study. MUCOS Pharma GmbH & Co, Geretsried, Germany 1990. Data on file
Singer F. Study No. MU-88202: therapeutic use of Wobenzym® in monoarticular activated arthrosis of knee joint. MUCOS Pharma GmbH & Co, Geretsried, Germany 1990. Data on file
Klein G. Study No. MU-88210: Wobenzym as base therapeutic in rheumatoid arthritis. Efficacy and tolerance. Arandomised, double blind study in parallel group versus auranofin. MUCOS Pharma GmbH & Co, Geretsried, Germany 1991. Data on file
Goubert LG. Study No. MU-696701: Wobenzym® N in the treatment of patients with gonarthritis. MUCOS Pharma GmbH & Co, Geretsried, Germany 1999. Data on file
Conradt C. Study No. MU-696702/B: re-analysis of efficacy of Wobenzym® N in rheumatic diseases by the mean of the epidemiological study: ‘proof of efficacy and harmlessness of Wobenzym® N in rheumatic diseases. A retrolective, cohort analysis in parallel groups (RetrospectTM) with oral nonsteroidal antirheumatics (NS AR) as reference therapy in the control group’. MUCOS Pharma GmbH & Co, Geretsried, Germany 2000.
Acknowledgements
The reviewers were supported by a grant from MUCOS Pharma GmbH & Co. for the clinical assessment of enzyme therapy.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Leipner, J., Iten, F. & Saller, R. Therapy with Proteolytic Enzymes in Rheumatic Disorders. BioDrugs 15, 779–789 (2001). https://doi.org/10.2165/00063030-200115120-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200115120-00001