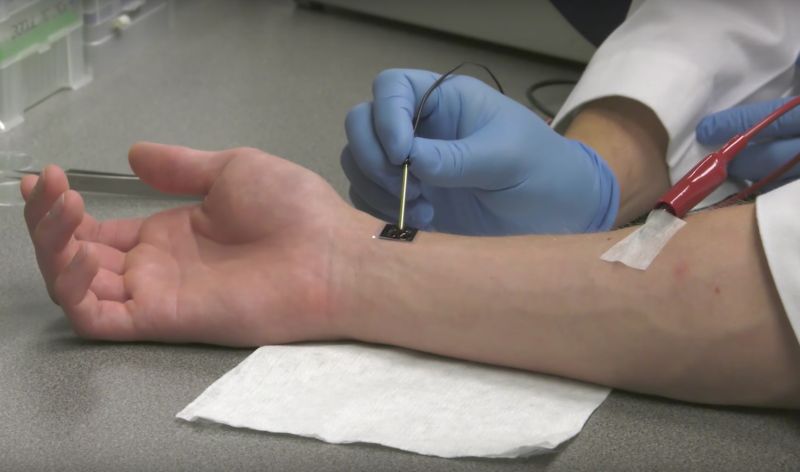
With a jolt from a tiny chip, humdrum skin cells may transform into medical mavericks.
A small electrical pulse blasts open tiny pores in cells and zaps in fragments of DNA or RNA loaded in the chip’s nanochannels. Those genetic deliveries then effectively reprogram the skin cells to act like other types of cells and repair damaged tissue. In early experiments on mice, researchers coaxed skin cells to act like brain cells. They also restored blood flow to a rodent’s injured limb by prompting skin cells to grow into new blood vessels.
The technology, published this week in Nature Nanotechnology, is still a long way from confirmed clinical applications in humans. But, the Ohio State researchers behind the chip are optimistic that it may one day perform myriad medical feats—including healing severe injuries, restoring diseased organs, erasing brain damage, and even turning back the clock on aging tissues.
The researchers, led by regenerative medicine expert Chandan Sen and biomolecular engineer L. James Lee, expect to begin clinical trials next year.
“The concept is very simple,” Lee said in a press statement. “As a matter of fact, we were even surprised how it worked so well. In my lab, we have ongoing research trying to understand the mechanism and do even better. So, this is the beginning, more to come.”
Their concept is similar to other cell-based regenerative therapies under development, but it skips some pesky steps. Some other methods explored by researchers—and dubious clinics—involve harvesting adult cells from patients, reprograming them to revert to stem cells, then injecting those cells back into patients, where they develop into a needed cell type.But this setup has snags. Researchers often use viruses to deliver the genetic elements that reprogram the cells, which have caused cancer in some animal studies. The method also requires a lot of manipulation of cells in lab, adding complications. It’s unclear if the suspect stem cell clinics are even successful at reprogramming cells.
Doctored cells
The method used by Lee, Sen, and colleagues ditches the need for a virus and for any cellular handling. The electrical pulse opens pores in cells that allow for direct genetic delivery—a process called electroporation. The researchers skipped the need to make stem cells by using preexisting methods of converting one cell type directly into a different one. Generally, this works by introducing bits of genetic material that code for gene regulators key to a specific cell type. Once delivered, these regulators can switch genes on or off so cells can start acting like the different cell type. Such a method has been worked out for creating liver, brain, and vascular cells from other cell types.
Finally, the researchers’ method also all takes place on a patch of skin on a living subject, potentially directly where it’s needed—no cell harvesting or lab manipulations are required. (That said, the researchers note that future therapies could use skin patches to generate specific cell types that can then be transferred to other locations in the body if needed.)
So far, the researchers have dabbled with making brain cells and vasculature cells from skin cells. In early experiments, their direct delivery proved effective at converting the cells. The researcher verified that the converted cells mirrored normal brain and vasculature cells' gene expression profiles—the pattern of genes they have turned on and off.
In their ultimate test, the researchers severed leg arteries in a handful of mice. Then a researcher placed over the injuries nanochips loaded with genetic ingredients for converting skin cells to vasculature cells. The conversion reached cells deep within the skin layers. After a week, the researchers saw more blood flow and less tissue death in the treated mice compared with control animals that weren’t treated.
Much work still needs to be done to test the idea and prove it's effective for certain treatments. But the researchers are optimistic. They conclude in the study that the technology “has the potential to ultimately enable the use of a patient’s own tissue as a prolific immunosurveilled bioreactor.”
Nature Nanotechnology, 2017. DOI: 10.1038/nnano.2017.134 (About DOIs).
reader comments
26