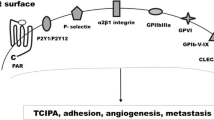
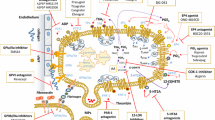
Abstract
Platelets act as multifunctional cells participating in immune response, inflammation, allergy, tissue regeneration, and lymphoangiogenesis. Among the best-established aspects of a role of platelets in non-hemostatic or thrombotic disorders, there is their participation in cancer invasion and metastasis. The interaction of many different cancer cells with platelets leads to platelet activation, and on the other hand platelet activation is strongly instrumental to the pro-carcinogenic and pro-metastatic activities of platelets. It is thus obvious that over the last years a lot of interest has focused on the possible chemopreventive effect of platelet-targeted pharmacologic treatments. This article gives an overview of the platelet-targeted pharmacologic approaches that have been attempted in the prevention of cancer development, progression, and metastasis, including the application of anti-platelet drugs currently used for cardiovascular disease and of new and novel pharmacologic strategies. Despite the fact that very promising results have been obtained with some of these approaches in pre-clinical models, with the exclusion of aspirin, clinical evidence of a beneficial effect of anti-platelet agents in cancer is however still largely missing. Future studies with platelet-targeted drugs in cancer must carefully deal with design issues, and in particular with the careful selection of patients, and/or explore novel platelet targets in order to provide a solution to the critical issue of the risk/benefit profile of long-term anti-platelet therapy in the prevention of cancer progression and dissemination.

Similar content being viewed by others
References
Simmons, D. L., Botting, R. M., & Hla, T. (2004). Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacological Reviews, 56(3), 387–437.
Iniguez, M. A., Cacheiro-Llaguno, C., Cuesta, N., Diaz-Munoz, M. D., & Fresno, M. (2008). Prostanoid function and cardiovascular disease. Archives of Physiology and Biochemistry, 114(3), 201–209.
Gresele, P., Deckmyn, H., Nenci, G. G., & Vermylen, J. (1991). Thromboxane synthase inhibitors, thromboxane receptor antagonists and dual blockers in thrombotic disorders. Trends in Pharmacological Sciences, 12(4), 158–163.
Nakahata, N. (2008). Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacological Therapy, 118(1), 18–35.
Vane, J. R. (1972). Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature: New Biology, 231(25), 232–235.
Roth, G. J., Stanford, N., & Majerus, P. W. (1975). Acetylation of prostaglandin synthase by aspirin. Proceedings of the National Academy of Sciences USA, 72(8), 3073–3076.
Gasic, G. J., Gasic, T. B., & Murphy, S. (1972). Anti-metastatic effect of aspirin. Lancet, 2(7783), 932–933.
Gasic, G. J., Gasic, T. B., & Stewart, C. C. (1968). Anti-metastatic effect associated with platelet reduction. Proceeding of the National Academy of Science USA, 61(1), 46–52.
Jaffe, B. M. (1974). Prostaglandin and cancer: an update. Prostaglandins, 6(6), 453–461.
Bennett, A., & Del Tacca, M. (1975). Proceedings: prostaglandins in human colonic carcinoma. Gut, 16(5), 409.
Reddy, B. S., Rao, C. V., Rivenson, A., & Kelloff, G. (1993). Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis, 14(8), 1493–1497.
Duperron, C., & Castonguay, A. (1997). Chemopreventive efficacies of aspirin and sulindac against lung tumorigenesis in A/J mice. Carcinogenesis, 18(5), 1001–1006.
Tian, Y., Ye, Y., Gao, W., et al. (2011). Aspirin promotes apoptosis in a murine model of colorectal cancer by mechanisms involving downregulation of IL-6-STAT3 signaling pathway. International Journal of Colorectal Disease, 26(1), 13–22.
Vad, N. M., Kudugunti, S. K., Wang, H., Bhat, G. J., & Moridani, M. Y. (2014). Efficacy of acetylsalicylic acid (aspirin) in skin B16-F0 melanoma tumor-bearing C57BL/6 mice. Tumour Biology, 35(5), 4967–4976.
Cathomas, G. (2014). PIK3CA in colorectal cancer. Frontiers in Oncology, 4, 1–4.
Okumura, H., Uchikado, Y., Setoyama, T., et al. (2014). Biomarkers for predicting the response of esophageal squamous cell carcinoma to neoadjuvant chemoradiation therapy. Surgery Today, 44(3), 421–428.
Reimers, M. S., Bastiaannet, E., Langley, R. E., et al. (2014). Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA International Medicine, 174(5), 732–739.
Henrich, K. O., Bauer, T., Schulte, J., et al. (2011). CAMTA1, a 1p36 tumor suppressor candidate, inhibits growth and activates differentiation programs in neuroblastoma cells. Cancer Research, 71(8), 3142–3151.
Mikami, J., Kurokawa, Y., Takahashi, T., et al. (2016). Antitumor effect of antiplatelet agents in gastric cancer cells: an in vivo and in vitro study. Gastric Cancer, 19(3), 817–826.
Guillem-Llobat, P., Dovizio, M., Bruno, A., et al. (2016). Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget, 7(22), 32462–32477.
Uluçkan, O., Eagleton, M. C., Floyd, D. H., et al. (2008). APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. Journal of Cellular Biochemistry, 104(4), 1311–1323.
Kune, G. A., Kune, S., & Watson, L. F. (1988). Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Research, 48(15), 4399–4404.
Algra, A. M., & Rothwell, P. M. (2012). Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncology, 13(5), 518–527.
Baron, J. A., Cole, B. F., & Sandler, R. S. (2003). A randomized trial of aspirin to prevent colorectal adenomas. New England Journal of Medicine, 348(10), 891–899.
Sandler, R. S., Halabi, S., & Baron, J. A. (2003). A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. New England Journal of Medicine, 348(10), 883–890.
Logan, R.F., Grainge, M.J., & Shepherd, V.C., et al.; (2008) ukCAP Trial Group. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology, 134(1), 29–38.
Benamouzig, R., Deyra, J., Martin, A., et al. (2003). Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology, 125(2), 328–336.
Cole, B. F., Logan, R. F., Halabi, S., et al. (2009). Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. Journal of the National Cancer Institute, 101(4), 256–266.
Burn, J., Bishop, D. T., Mecklin, J. P., & CAPP2 Investigators. (2008). Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. New England Journal of Medicine, 359(24), 2567–2578.
Cooke, N. M., Spillane, C. D., Sheils, O., O'Leary, J., & Kenny, D. (2015). Aspirin and P2Y12 inhibition attenuate platelet-induced ovarian cancer cell invasion. BMC Cancer, 15, 627.
Antithrombotic Trialists’ Collaboration. (2002). Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. British Medical Journal, 324(7329), 71–86.
Gresele, P. (2013). Antiplatelet agents in clinical practice and their haemorrhagic risk. Blood Transfusion, 11(3), 349–356.
Stürmer, T., Glynn, R. J., Lee, I. M., Manson, J. E., Buring, J. E., & Hennekens, C. H. (1998). Aspirin use and colorectal cancer: post-trial follow-up data from the Physicians’ Health Study. Annals of Internal Medicine, 128(9), 713–720.
Rothwell, P. M., Wilson, M., & Elwin, C. E. (2010). Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet, 376(9754), 1741–1750.
Rothwell, P. M., Fowkes, F. G., Belch, J. F., Ogawa, H., Warlow, C. P., & Meade, T. W. (2011). Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet, 377(9759), 31–41.
Rothwell, P. M., Wilson, M., Price, J. F., Belch, J. F., Meade, T. W., & Mehta, Z. (2012). Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet, 379(9826), 1591–1601.
Rothwell, P. M., Price, J. F., Fowkes, F. G., et al. (2012). Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet, 379(9826), 1602–1612.
Thun, M. J., Jacobs, E. J., & Patrono, C. (2012). The role of aspirin in cancer prevention. Nature Reviews Clinical Oncology, 9(5), 259–267.
Chubak, J., Whitlock, E. P., Williams, S. B., et al. (2016). Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 164(12), 814–825.
Gresele, P., Momi, S., & Falcinelli, E. (2011). Anti-platelet therapy: phosphodiesterase inhibitors. British Journal of Clinical Pharmacology, 72(4), 634–646.
Faxon, D.P., Creager, M.A., Smith, S.C., et al., (2004) American Heart Association. Atherosclerotic vascular disease conference: executive summary: atherosclerotic vascular disease conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation, 109(21), 2595–2604.
Murata, K., Kameyama, M., Fukui, F., et al. (1999). Phosphodiesterase type III inhibitor, cilostazol, inhibits colon cancer cell motility. Clinical and Experimental Metastasis, 17(6), 525–530.
Uzawa, K., Kasamatsu, A., Baba, T., et al. (2013). Targeting phosphodiesterase 3B enhances cisplatin sensitivity in human cancer cells. Cancer Medicine, 2(1), 40–49.
Okoshi, H., Hakomori, S., Nisar, M., et al. (1991). Cell membrane signaling as target in cancer therapy II: inhibitory effect of N,N,N-trimethylsphingosine on metastatic potential of murine B16 melanoma cell line through blocking of tumor cell-dependent platelet aggregation. Cancer Research, 51(22), 6019–6025.
Inufusa, H., Adachi, T., Nakamura, M., Shindo, K., Yasutomi, M., & Kimura, Y. (1995). Inhibition of experimental metastasis of human adenocarcinoma by cilostazol, a platelet phosphodiesterase inhibitor. Oncology Report, 2(6), 1079–1083.
Akcan, A., Kucuk, C., Ok, E., Canoz, O., Muhtaroglu, S., et al. (2006). The effect of amrinone on liver regeneration in experimental hepatic resection model. The Journal of Surgical Research, 130(1), 66–72.
Savai, R., Pullamsetti, S. S., Banat, G. A., et al. (2010). Targeting cancer with phosphodiesterase inhibitors. Expert Opinion on Investigational Drugs, 19(1), 117–131.
Strowitzki, M. J., Dold, S., von Heesen, M., et al. (2014). The phosphodiesterase 3 inhibitor cilostazol does not stimulate growth of colorectal liver metastases after major hepatectomy. Clinical and Experimental Metastasis, 31(7), 795–803.
Gresele, P., Zoja, C., Deckmyn, H., Arnout, J., Vermylen, J., & Verstraete, M. (1983). Dipyridamole inhibits platelet aggregation in whole blood. Thrombosis and Haemostasis, 50(4), 852–856.
Gresele, P., Arnout, J., Deckmyn, H., & Vermylen, J. (1986). Mechanism of the antiplatelet action of dipyridamole in whole blood: modulation of adenosine concentration and activity. Thrombosis and Haemostasis, 55(1), 12–18.
Gresele, P., Arnout, J., & Vermylen, J. (1987). Dipyridamole inhibits leukotriene B4 synthesis. Thrombosis and Haemostasis, 57(2), 235.
Deckmyn, H., Gresele, P., Arnout, J., Todisco, A., & Vermylen, J. (1984). Prolonging prostacyclin production by nafazatrom or dipyridamole. Lancet, 2(8399), 410–411.
Tzanakakis, G. N., Agarwal, K. C., & Vezeridis, M. P. (1993). Prevention of human pancreatic cancer cell-induced hepatic metastasis in nude mice by dipyridamole and its analog RA-233. Cancer, 71(8), 2466–2471.
Desai, P. B., Duan, J., Sridhar, R., & Damle, B. D. (1997). Reversal of doxorubicin resistance in multidrug resistant melanoma cells in vitro and in vivo by dipyridamole. Methods and Findings in Experimental Clinical Pharmacology, 19(4), 231–239.
Spano, D., Marshall, J. C., Marino, N., et al. (2013). Dipyridamole prevents triple-negative breast-cancer progression. Clinical and Experimental Metastasis, 30(1), 47–68.
Goda, A. E., Yoshida, T., Horinaka, M., et al. (2008). Mechanisms of enhancement of TRAIL tumoricidal activity against human cancer cells of different origin by dipyridamole. Oncogene, 27(24), 3435–3445.
Shalinsky, D. R., Andreeff, M., & Howell, S. B. (1990). Modulation of drug sensitivity by dipyridamole in multidrug resistant tumor cells in vitro. Cancer Research, 50(23), 7537–7543.
Rhodes, E. L., Misch, K. J., Edwards, J. M., & Jarrett, P. E. (1985). Dipyridamole for treatment of melanoma. Lancet, 1(8430), 693.
Kohnoe, S., Maehara, Y., Takahashi, I., Emi, Y., Baba, H., & Sugimachi, K. (1998). Treatment of advanced gastric cancer with 5-fluorouracil and cisplatin in combination with dipyridamole. International Journal of Oncology, 13(6), 1203–1206.
Todd, K. E., Gloor, B., Lane, J. S., Isacoff, W. H., & Reber, H. A. (1998). Resection of locally advanced pancreatic cancer after downstaging with continuous-infusion 5-fluorouracil, mitomycin-C, leucovorin, and dipyridamole. Journal of Gastrointestinal Surgery, 2(2), 159–166.
Isacoff, W. H., Bendetti, J. K., Barstis, J. J., Jazieh, A. R., Macdonald, J. S., & Philip, P. A. (2007). Phase II trial of infusional fluorouracil, leucovorin, mitomycin, and dipyridamole in locally advanced unresectable pancreatic adenocarcinoma: SWOG S9700. Journal of Clinical Oncology, 25(13), 1665–1669.
Cusack, N. J., & Hourani, S. M. O. (2000). Platelet P2 receptors: from curiosity to clinical targets. Journal of Autonomous Nervous System, 81(1–3), 37–43.
Burnstock, G. (1972). Purinergic nerves. Pharmacological Reviews, 24(3), 509–581.
Hollopeter, G., Jantzen, H. M., Vincent, D., et al. (2001). Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature, 409(6817), 202–207.
Andre, P., Delaney, S. M., La Rocca, T., et al. (2003). P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. Journal of Clinical Investigation, 112(3), 398–406.
Dangelmaier, C., Jin, J., Smith, J. B., & Kunapuli, S. P. (2001). Potentiation of thromboxane A2-induced platelet secretion by Gi signaling through the phosphoinositide-3 kinase pathway. Thrombosis and Haemostasis, 85(2), 341–348.
Dorsam, R. T., Kim, S., Jin, J., & Kunapuli, S. P. (2002). Coordinated signaling through both G12/13 and G(i) pathways is sufficient to activate GPIIb/IIIa in human platelets. Journal of Biological Chemistry, 277(49), 47588–47595.
Dorsam, R. T., & Kunapuli, S. P. (2004). Central role of the P2Y12 receptor in platelet activation. Journal of Clinical Investigation, 113(3), 340–345.
van Gestel, M. A., Heemskerk, J. W., Slaaf, D. W., et al. (2003). In vivo blockade of platelet ADP receptor P2Y12 reduces embolus and thrombus formation but not thrombus stability. Arteriosclerosis Thrombosis and Vascular Biology, 23(3), 518–523.
Woulfe, D., Jiang, H., Mortensen, R., Yang, J., & Brass, L. F. (2002). Activation of Rap1B by G(i) family members in platelets. Journal of Biological Chemistry, 277(26), 23382–23390.
Gachet, C. (2012). P2Y12 receptors in platelets and other hematopoietic and non-hematopoietic cells. Purinergic Signal, 8(3), 609–619.
Di Virgilio, F. (2012). Purines, purinergic receptors, and cancer. Cancer Research, 72(21), 5441–5447.
Aymeric, L., Apetoh, L., Ghiringhelli, F., et al. (2010). Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Research, 70(3), 855–858.
Boukerche, H., Berthier-Vergnes, O., Penin, F., et al. (1994). Human melanoma cell lines differ in their capacity to release ADP and aggregate platelets. British Journal of Haematology, 87(4), 763–772.
Ordinas, A., Díaz-Ricart, M., Almirall, L., & Bastida, E. (1990). The role of platelets in cancer metastasis. Blood Coagulation and Fibrinolysis, 1(6), 707–711.
Wang, Y., Sun, Y., Li, D., et al. (2013). Platelet P2Y12 is involved in murine pulmonary metastasis. PloS One, 8(11), e80780.
Coupland, L. A., & Parish, C. R. (2014). Platelets, selectins, and the control of tumor metastasis. Seminars in Oncology, 41(3), 422–434.
Johansson, J., Tabor, V., Wikell, A., Jalkanen, S., & Fuxe, J. (2015). TGF-β1-induced epithelial-mesenchymal transition promotes monocyte/macrophage properties in breast cancer cells. Frontiers in Oncology, 5, 3.
Contractor, H., & Ruparelia, N. (2012). Advances in antiplatelet therapy for acute coronary syndromes. Postgraduate Medical Journal, 88(1041), 391–396.
Collet, J. P., Hulot, J. S., Pena, A., et al. (2009). Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet, 373(9660), 309–317.
Herbert, J. M., & Savi, P. (2003). P2Y12, a new platelet ADP receptor, target of clopidogrel. Seminars in Vascular Medicine, 3(2), 113–122.
Kazui, M., Nishiya, Y., Ishizuka, T., et al. (2010). Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metabolism and Disposition, 38(1), 92–99.
Pereillo, J. M., Maftouh, M., Andrieu, A., et al. (2002). Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metabolism and Disposition, 30(11), 1288–1295.
Bambace, N. M., Levis, J. E., & Holmes, C. E. (2010). The effect of P2Y-mediated platelet activation on the release of VEGF and endostatin from platelets. Platelets, 21(2), 85–93.
Klein-Soyer, C., Céraline, J., Orvain, C., de la Salle, C., Bergerat, J. P., & Cazenave, J. P. (1997). Angiogenesis inhibitor SR 25989 upregulates thrombospondin-1 expression in human vascular endothelial cells and foreskin fibroblasts. Biology of the Cell, 89(4), 295–307.
Ma, H., Hara, A., Xiao, C. Y., et al. (2001). Increased bleeding tendency and decreased susceptibility to thromboembolism in mice lacking the prostaglandin E2 receptor subtype EP3. Circulation, 104(10), 1176–1180.
Sitia, G., Aiolfi, R., Di Lucia, P., et al. (2012). Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proceeding of the National Academy of Sciences U S A, 109(32), E2165–E2172.
Pandey, A., Sarangi, S., Chien, K., et al. (2014). Anti-platelet agents augment cisplatin nanoparticle cytotoxicity by enhancing tumor vasculature permeability and drug delivery. Nanotechnology, 25(44), 445101.
Roop, R. P., Naughton, M. J., Van Poznak, C., et al. (2013). A randomized phase II trial investigating the effect of platelet function inhibition on circulating tumor cells in patients with metastatic breast cancer. Clinical Breast Cancer, 13(6), 409–415.
Choe, K. S., Correa, D., Jani, A. B., & Liauw, S. L. (2010). The use of anticoagulants improves biochemical control of localized prostate cancer treated with radiotherapy. Cancer, 116(7), 1820–1826.
Hicks, B. M., Murray, L. J., Hughes, C., & Cardwell, C. R. (2015). Clopidogrel use and cancer-specific mortality: a population-based cohort study of colorectal, breast and prostate cancer patients. Pharmacoepidemiological Drug Safety, 24(8), 830–840.
Husted, S., Emanuelsson, H., Heptinstall, S., et al. (2006). Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. European Heart Journal, 27(9), 1038–1047.
Teng, R., Oliver, S., Hayes, M. A., & Butler, K. (2010). Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metabolism and Disposable, 38(9), 1514–1521.
Gebremeskel, S., LeVatte, T., Liwski, R. S., Johnston, B., & Bezuhly, M. (2015). The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. International Journal of Cancer, 136(1), 234–240.
Bonaca, M. P., Bhatt, D. L., Cohen, M. P. H., et al. (2015). Long-term use of ticagrelor in patients with prior myocardial infarction. New England Journal of Medicine, 372(19), 1791–1800.
Serebruany, V. L., Cherepanov, V., Cabrera-Fuentes, H. A., & Kim, M. H. (2015). Solid cancers after antiplatelet therapy: confirmations, controversies, and challenges. Thrombosis and Haemostasis, 114(6), 1104–1112.
Serebruany, V. L., Dinicolantonio, J. J., Can, M. M., Pershukov, I. V., & Kuliczkowski, W. (2013). Gastrointestinal adverse events after dual antiplatelet therapy: clopidogrel is safer than ticagrelor, but prasugrel data are lacking or inconclusive. Cardiology, 126(1), 35–40.
Unger, E. F. (2009). Weighing benefits and risks—the FDA’s review of prasugrel. New England Journal of Medicine, 361(10), 942–945.
Floyd, J. S., & Serebruany, V. L. (2010). Prasugrel as a potential cancer promoter: review of the unpublished data. Archives of Internal Medicine, 170(12), 1078–1080.
Roe, M.T., Cyr, D.D., Eckart, D., et al.; (2016) TRILOGY ACS Investigators. Ascertainment, classification, and impact of neoplasm detection during prolonged treatment with dual antiplatelet therapy with prasugrel vs clopidogrel following acute coronary syndrome. European Heart Journal, 37(4), 412–422.
Mauri, L., Kereiakes, D.J., Yeh, R.W., et al. (2014). DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. New England Journal of Medicine, 371(23), 2155–2166.
Kotronias, R. A., Kwok, C. S., Wong, C. W., Kinnaird, T., Zaman, A., & Mamas, M. A. (2017). Cancer event rate and mortality with thienopyridines: a systematic review and meta-analysis. Drug Safety, 40(3), 229–240.
Leader, A., Zelikson-Saporta, R., Pereg, D., et al. (2017). The effect of combined aspirin and clopidogrel treatment on cancer incidence. The American Journal of Medicine. doi:10.1016/j.amjmed.2017.01.022.
Shattil, S. J., Kashiwagi, H., & Pampori, N. (1998). Integrin signaling: the platelet paradigm. Blood, 91(8), 2645–2657.
Schrör, K., & Weber, A. (2003). Comparative pharmacology of GP IIb/IIIa antagonists. Journal of Thrombosis and Thrombolysis, 15(5), 71–80.
Salame, M., Verheye, S., More, R., King 3rd, S. B., & Chronos, N. (1999). GPIIbIIIa inhibitors as adjunctive therapy in acute myocardial infarction. International Journal of Cardiology, 69(3), 231–236.
Ahrens, I., Bode, C., & Zirlik, A. (2014). Anticoagulation during and after acute coronary syndrome. Hämostaseologie, 34(1), 72–77.
Clezardin, P., Drouin, J., Morel-Kopp, M. C., et al. (1993). Role of platelet membrane glycoproteins Ib/IX and IIb/IIIa, and of platelet alpha-granule proteins in platelet aggregation induced by human osteosarcoma cells. Cancer Research, 53(19), 4695–4700.
Santos-Martinez, M. J., Medina, C., Jurasz, P., & Radomski, M. W. (2008). Role of metalloproteinases in platelet function. Thrombosis Research, 121(4), 535–542.
Bakewell, S. J., Nestor, P., Prasad, S., et al. (2003). Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proceedings of the National Academy of Science U S A, 100(24), 14205–14210.
Liu, Z., Wang, F., & Chen, X. (2008). Integrin alpha(v)beta(3)-targeted cancer therapy. Drug Development Research, 69(6), 329–339.
Bennett, J. S., Chan, C., Vilaire, G., Mousa, S. A., & DeGrado, W. F. (1997). Agonist-activated alphavbeta3 on platelets and lymphocytes binds to the matrix protein osteopontin. Journal of Biological Chemistry, 272(13), 8137–8140.
Wilder, R. L. (2002). Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Annals of Rheumatisms Disease, 61(Suppl 2), 96–99.
Brooks, P. C., Clark, R. A., & Cheresh, D. A. (1994). Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science, 264(5158), 569–571.
Kumar, C. C. (2003). Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Current Drug Targets, 4(2), 123–131.
Weber, M. R., Zuka, M., Lorger, M., et al. (2016). Activated tumor cell integrin αvβ3 cooperates with platelets to promote extravasation and metastasis from the blood stream. Thrombosis Research, 140(Suppl 1), S27–S36.
Tesfamariam, B. (2016). Involvement of platelets in tumor cell metastasis. Pharmacological. Therapy, 157, 112–119.
Lonsdorf, A. S., Kramer, B. F., Fahrleitner, M., et al. (2012). Engagement of alpha(IIb)beta(3) (GPIIb/IIIa) with alphanubeta3 integrin mediates interaction of melanoma cells with platelets: a connection to hematogenous metastasis. Journal of Biological Chemistry, 287(3), 2168–2178.
Gomes, N., Vassy, J., Lebos, C., Arbeille, B., Legrand, C., & Fauvel-Lafeve, F. (2004). Breast adenocarcinoma cell adhesion to the vascular subendothelium in whole blood and under flow conditions: effects of alphavbeta3 and alphaIIbbeta3 antagonists. Clinical and Experimental Metastasis, 21(6), 553–561.
Harris, T. D., Kalogeropoulos, S., Nguyen, T., et al. (2003). Design, synthesis, and evaluation of radiolabeled integrin alpha v beta 3 receptor antagonists for tumor imaging and radiotherapy. Cancer Biotherapy and Radiopharmaceuticals, 18(4), 627–641.
Amirkhosravi, A., Mousa, S. A., Amaya, M., et al. (2003). Inhibition of tumor cell-induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thrombosis and Haemostasis, 90(3), 549–554.
Sheu, J. R., Lin, C. H., Chung, J.l., Teng, C. M., & Huang, T. F. (1992). Triflavin, an Arg-Gly-Asp containing snake venom peptide, inhibits aggregation of human platelets induced by human hepatoma cell line. Thrombosis Research, 66(6), 679–691.
Chiang, H. S., Swaim, M. W., & Huang, T. F. (1994). Characterization of platelet aggregation induced by human colon adenocarcinoma cells and its inhibition by snake venom peptides, trigramin and rhodostomin. British Journal of Haematology, 87(2), 325–331.
Borsig, L., Wong, R., Feramisco, J., Nadeau, D. R., Varki, N. M., & Varki, A. (2001). Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proceedings of the National Academy of Science U S A, 98(6), 3352–3357.
Sobel, M., Fish, W. R., Toma, N., et al. (2001). Heparin modulates integrin function in human platelets. Journal of Vascular Surgery, 33(3), 587–594.
Zhang, C., Liu, Y., Gao, Y., et al. (2009). Modified heparins inhibit integrin alpha(IIb)beta(3) mediated adhesion of melanoma cells to platelets in vitro and in vivo. International Journal of Cancer, 125(9), 2058–2065.
Gutheil, J. C., Campbell, T. N., Pierce, P. R., et al. (2000). Targeted antiangiogenic therapy for cancer using vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clinical and Cancer Research, 6(8), 3056–3061.
Patel, S., Jenkins, J., Papadopolous, N., et al. (2001). Pilot study of vitaxin—an angiogenesis inhibitor—in patients with advanced leiomyosarcomas. Cancer, 92(5), 1347–1348.
Posey, J., Khazaeli, M., Del Grosso, A., et al. (2001). A pilot trial of vitaxin, a humanized anti-vitronectin receptor (anti-αvβ3) antibody in patients with metastatic cancer. Cancer Biotherapy and Radiopharmaceuticals, 16(2), 125–132.
Cai, W., Wu, Y., Chen, K., Cao, Q., Tice, D., & Chen, X. (2006). In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin αvβ3. Cancer Research, 66(19), 9673–9681.
Hersey, P., Sosman, J., O'Day, S., et al. (2010). A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin αvβ3, +/− dacarbazine in patients with stage IV metastatic melanoma. Cancer, 116(6), 1526–1534.
Delbaldo, C., Raymond, E., Vera, K., et al. (2008). Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against αvβ3 integrin receptor, in patients with advanced solid tumors. Investigational New Drugs, 26(1), 35–43.
Dyke, C. M. (1999). Safety of glycoprotein IIb-IIIa inhibitors: a heart surgeon’s perspective. American Heart Journal, 138(4 pt 2), 307–316.
Gulba, D. C., Huber, K., Moll, S., & Dietz, R. (1998). Platelet inhibition: new agents, new strategies, new trials. Fibrinolysis and Proteolysis, 12(Suppl2), 13–23.
Tam, S., Sassoli, P., Jordan, R., & Nakada, M. (1998). Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of lycoprotein IIb/IIIa αvβ3 integrins. Circulation, 98(11), 1085–1091.
Antoniucci, D. (2007). Differences among GP IIb/IIIa inhibitors: different clinical benefits in non-ST-segment elevation acute coronary syndrome percutaneous coronary intervention patients. European Heart Journal, 9, A32–A36.
Casserly, I., & Topol, E. (2002). Glycoprotein IIb/IIIa-antagonists—from bench to practice. Cellular and Molecular Life Sciences, 59(3), 478–500.
Amirkhosravi, A., Amaya, M., Siddiqui, F., Biggerstaff, J. P., Meyer, T. V., & Francis, J. L. (1999). Blockade of GPIIb/IIIa inhibits the release of vascular endothelial growth factor (VEGF) from tumor cell-activated platelets and experimental metastasis. Platelets, 10(5), 285–292.
Trikha, M., Zhou, Z., Timar, J., et al. (2002). Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Research, 62(10), 2824–2833.
Tcheng, J. (2000). Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. American Heart Journal, 139(2 pt 2), S38–S45.
Kononczuk, J., Surazynski, A., Czyzewska, U., et al. (2015). αIIbβ3-integrin ligands: abciximab and eptifibatide as proapoptotic factors in MCF-7 human breast cancer cells. Current Drug Targets, 16(13), 1429–1437.
Karlheinz, P. (2005). Antiplatelet drugs. In M. S. Runge (Ed.), Principles of molecular cardiology (Vol. 105, pp. 203–218). Totowa: Human Press.
Peter, K. (2005). Principles of molecular cardiology. Totowa: Humana Press.
Davì, G., Santilli, F., & Vazzana, N. (2012). Thromboxane receptors antagonists and/or synthase inhibitors. Handbook of Experimental Pharmacology, 210, 261–286.
Honn, K. V. (1983). Inhibition of tumor cell metastasis by modulation of the vascular prostacyclin/thromboxane A2 system. Clinical and Experimental Metastasis, 1(2), 103–114.
Ogletree, M. L. (1987). Overview of physiological and pathophysiological effects of thromboxane A2. Federation Proceedings, 46(1), 133–138.
Nie, D., Lamberti, M., Zacharek, A., et al. (2000). Thromboxane A2 regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochemical and Biophysical Research Communication, 267, 245–251.
de Leval, X., Benoit, V., Delarge, J., et al. (2003). Pharmacological evaluation of the novel thromboxane modulator BM-567 (II/II). Effects of BM-567 on osteogenic sarcoma-cell-induced platelet aggregation. Prostaglandins and Leukotriene Essential Fatty Acids, 68(1), 55–59.
Mehta, P., Lawson, D., Ward, M. B., Lee-Ambrose, L., & Kimura, A. (1986). Effects of thromboxane A2 inhibition on osteogenic sarcoma cell-induced platelet aggregation. Cancer Research, 46(10), 5061–5063.
Yokoyama, I., Hayashi, S., Kobayashi, T., et al. (1995). Prevention of experimental hepatic metastasis with thromboxane synthase inhibitor. Research and Experimental Medicine, 195(4), 209–215.
Vezza, R., Roberti, R., Nenci, G. G., & Gresele, P. (1993). Prostaglandin E2 potentiates platelet aggregation by priming protein kinase C. Blood, 82(9), 2704–2713.
Fabre, J. E., Nguyen, M. T., Athirakul, K., et al. (2001). Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. Journal of Clinical Investigation, 107(5), 603–610.
Gross, S., Tilly, P., Hentsch, D., et al. (2007). Vascular-wall produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. Journal of Experimental Medicine, 204(2), 311–320.
Gresele, P., Blockmans, D., Deckmyn, H., & Vermylen, J. (1988). Adenylate cyclase activation determines the effect of thromboxane synthase inhibitors on platelet aggregation in vitro. Comparison of platelets from responders and nonresponders. Journal of Pharmacological and Experimental Therapeutics, 246(1), 301–307.
Singh, J., Zeller, W., Zhou, N., et al. (2009). Antagonists of the EP3 receptor for prostaglandin E2 are novel antiplatelet agents that do not prolong bleeding. ACS Chemical Biology, 4(2), 115–126.
Fox, S. C., May, J. A., Johnson, A., et al. (2013). Effects on platelet function of an EP3 receptor antagonist used alone and in combination with a P2Y12 antagonist both in vitro and ex vivo in human volunteers. Platelets, 24(5), 392–400.
Honn, K. V., Cicone, B., & Skoff, A. (1981). Prostacyclin: a potent antimetastatic agent. Science, 212(4500), 1270–1272.
Dogne, J. M., Hanson, J., & Pratico, D. (2005). Thromboxane, prostacyclin and isoprostanes: therapeutic targets in atherogenesis. Trends in Pharmacological Science, 26(12), 639–644.
Fetalvero, K. M., Martin, K. A., & Hwa, J. (2007). Cardioprotective prostacyclin signaling in vascular smooth muscle. Prostaglandins and Other Lipid Mediators, 82(1–4), 109–118.
Keith, R. L., & Geraci, M. W. (2006). Prostacyclin in lung cancer. J Thoracic Oncology, 1(6), 503–505.
Grommes, C., Landreth, G. E., & Heneka, M. T. (2004). Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncology, 5(7), 419–429.
Nemenoff, R. A., Meyer, A. M., Hudish, T. M., et al. (2008). Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator-activated receptor gamma. Cancer Prevention Research, 1(5), 349–356.
He, P., Borland, M. G., Zhu, B., et al. (2008). Effect of ligand activation of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) in human lung cancer cell lines. Toxicology, 254(1–2), 112–117 26.
Pedchenko, T. V., Gonzalez, A. L., Wang, D., DuBois, R. N., & Massion, P. P. (2008). Peroxisome proliferator-activated receptor beta/delta expression and activation in lung cancer. American Journal of Respiratory Cell and Molecular Biology, 39(6), 689–696.
Menter, D. G., Onoda, J. M., Taylor, J. D., & Honn, K. V. (1984). Effects of prostacyclin on tumor cell-induced platelet aggregation. Cancer Research, 44(2), 450–456.
Menter, D. G., Onoda, J. M., Moilanen, D., Sloane, B. F., Taylor, J. D., & Honn, K. V. (1987). Inhibition by prostacyclin of the tumor cell-induced platelet release reaction and platelet aggregation. Journal of the National Cancer Institute, 78(5), 961–969.
Honn, V. H., Cicone, B., & Skoff, A. (1980). Prostacyclin: a potent antimetastatic agent. Science, 212(4500), 1270–1272.
Cuneo, K. C., Fu, A., Osusky, K. L., & Geng, L. (2007). Effects of vascular endothelial growth factor receptor inhibitor SU5416 and prostacyclin on murine lung metastasis. Anti-Cancer Drugs, 18(3), 349–355.
Keith, R. L., Miller, Y. E., Hoshikawa, Y., et al. (2002). Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Research, 62(3), 734–740.
Keith, R. L., Miller, Y. E., Hudish, T. M., et al. (2004). Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Research, 64(16), 5897–5904.
Honn, K. V., Meyer, J., Neagos, G., Henderson, T., Westley, C., & Ratanatharathorn, V. (1982). Control of tumor growth and metastasis with prostacyclin and thromboxane synthetase inhibitors: evidence for a new antitumor and antimetastatic agent (BAY G 6575). In G. A. Jamieson (Ed.), Interaction of platelets and tumor cells (pp. 295–331). New York: Alan R. Uss.
Bren-Mattison, Y., Van Putten, V., Chan, D., Winn, R., Geraci, M. W., & Nemenoff, R. A. (2005). Peroxisome proliferator-activated receptor-gamma (PPAR(gamma)) inhibits tumorigenesis by reversing the undifferentiated phenotype of metastatic non-small-cell lung cancer cells (NSCLC). Oncogene, 24, 1412–1422.
Keith, R. L., Blatchford, P. J., Kittelson, J., et al. (2011). Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prevention Research, 4(6), 793–802.
Mascaux, C., Feser, W. J., Lewis, M. T., et al. (2013). Endobronchial miRNAs as biomarkers in lung cancer chemoprevention. Cancer Prevention Research, 6(2), 100–108.
Gibbins, J. M., Okuma, M., Farndale, R., Barnes, M., & Watson, S. P. (1997). Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor gamma-chain. FEBS Letters, 413, 255–259.
Nieswandt, B., Bergmeier, W., Schulte, V., Rackebrandt, K., Gessner, J. E., & Zirngibl, H. (2000). Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRgamma chain. Journal of Biological Chemistry, 275(31), 23998–24002.
Nieswandt, B., Schulte, V., Bergmeier, W., et al. (2001). Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. Journal of Experimental Medicine, 193(4), 459–469.
Nieswandt, B., Brakebusch, C., Bergmeier, W., et al. (2001). Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO Journal, 20(9), 2120–2130.
Clemetson, J. M., Polgar, J., Magnenat, E., Wells, T. N., & Clemetson, K. J. (1999). The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcalphaR and the natural killer receptors. Journal of Biological Chemistry, 274(8), 29019–29024.
Jandrot-Perrus, M., Busfield, S., Lagrue, A. H., et al. (2000). Cloning, characterization, and functional studies of human and mouse glycoprotein VI: a platelet-specific collagen receptor from the immunoglobulin superfamily. Blood, 96(5), 1798–1807.
Moroi, M., Jung, S. M., Okuma, M., & Shinmyozu, K. (1989). A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. Journal of Clinical Investigation, 84(5), 1440–1445.
Watson, S. P., Asazuma, N., Atkinson, B., et al. (2001). The role of ITAM- and ITIM-coupled receptors in platelet activation by collagen. Thrombosis and Haemostasis, 86, 276–288.
Jain, S., Russell, S., & Ware, J. (2009). Platelet glycoprotein VI facilitates experimental lung metastasis in syngenic mouse models. Journal of Thrombosis and Haemostasis, 7(10), 1713–1717.
Ungerer, M., Rosport, K., Bultmann, A., et al. (2011). Novel antiplatelet drug revacept (dimeric glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation, 123(17), 891–1899.
Dovizio, M., Maier, T. J., Alberti, S., et al. (2013). Pharmacological inhibition of platelet–tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Molecular Pharmacology, 84(1), 25–40.
Nangia-Makker, P., Balan, V., & Raz, A. (2008). Regulation of tumor progression by extracellular galectin-3. Cancer Microenvironment, 1(1), 43–51.
Coughlin, S. R. (2000). Thrombin signalling and protease-activated receptors. Nature, 407(6801), 258–264.
Krishnaswamy, S. (2013). The transition of prothrombin to thrombin. Journal of Thrombosis and Haemostasis, 11(Suppl 1), 265–276.
Daniel, T., Gibbs, V. C., Milfay, D., et al. (1986). Thrombin stimulates c-sis gene expression in microvascular endothelial cells. Journal of Biological Chemistry, 261(21), 9579–9582.
DeMichele, M., & Minnear, F. (1992). Modulation of vascular endothelial permeability by thrombin. Seminars in Thrombosis and Hemostasis, 18(3), 287–295.
Wojtukiewicz, M. Z., Tang, D. G., Ciarelli, J. J., et al. (1993). Thrombin increases the metastatic potential of tumor cells. International Journal of Cancer, 54(5), 793–806.
Nierodzik, M., Kajumo, F., & Karpatkin, S. (1992). Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and metastasis in vivo. Cancer Research, 52(12), 3267–3272.
Chen, L. B., & Buchanan, J. M. (1975). Mitogenic activity of blood components. I. Thrombin and prothrombin. Proceedings of the National Academy of Science U S A, 72(1), 131–135.
Szaba, F. M., & Smiley, S. T. (2002). Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood, 99, 1053–1059.
Sugama, Y., Tiruppathi, C., Offakidevi, K., Andersen, T. T., Fenton 2nd, J. W., & Malik, A. B. (1992). Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: a mechanism for stabilizing neutrophil adhesion. Journal of Cell Biology, 119(4), 935–944.
Chiang, H. S., Yang, R. S., & Huang, T. F. (1996). Thrombin enhances the adhesion and migration of human colon adenocarcinoma cells via increased beta 3-integrin expression on the tumour cell surface and their inhibition by the snake venom peptide, rhodostomin. British Journal of Cancer, 73(7), 902–908.
Radjabi, A. R., Sawada, K., Jagadeeswaran, S., et al. (2008). Thrombin induces tumor invasion through the induction and association of matrix metalloproteinase-9 and beta1-integrin on the cell surface. Journal of Biological Chemistry, 283(5), 2822–2834.
Nierodzik, M. L., & Karpatkin, S. (2006). Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell, 110(5), 355–362.
Coughlin, S. R. (2005). Protease-activated receptors in hemostasis, thrombosis and vascular biology. Journal of Thrombosis and Haemostasis, 3(8), 1800–1814.
Huang, Z., Miao, X., Luan, Y., et al. (2015). PAR1-stimulated platelet releasate promotes angiogenic activities of endothelial progenitor cells more potently than PAR4-stimulated platelet releasate. Thrombosis and Haemostasis, 13(3), 465–476.
Sedda, S., Marafini, I., Caruso, R., Pallone, F., & Monteleone, G. (2014). Proteinase activated-receptors-associated signaling in the control of gastric cancer. World Journal of Gastroenterology, 20(34), 11977–11984.
Albrektsen, T., Sorensen, B. B., Hjorto, G. M., Fleckner, J., Rao, L. V., & Petersen, L. C. (2007). Transcriptional program induced by factor VIIa tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. Journal of Thrombosis and Haemostasis, 5(8), 1588–1597.
Zhou, W., Hashimoto, K., Goleniewska, K., et al. (2007). Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. Journal of Immunology, 178(2), 702–710.
Otsuki, T., Fujimoto, D., Hirono, Y., Goi, T., & Yamaguchi, A. (2014). Thrombin conducts epithelial mesenchymal transition via protease activated receptor 1 in human gastric cancer. International Journal of Oncology, 45(6), 2287–2294.
Fujimoto, D., Hirono, Y., Goi, T., Katayama, K., Matsukawa, S., & Yamaguchi, A. (2010). The activation of proteinase-activated receptor-1 (PAR1) mediates gastric cancer cell proliferation and invasion. Biomedical Central Cancer, 10, 443–458.
Uzunoglu, F. G., Yavari, N., Bohn, B. A., et al. (2013). C-X-C motif receptor 2, endostatin and proteinase-activated receptor 1 polymorphisms as prognostic factors in NSCLC. Lung Cancer, 81(1), 123–129.
Kaufmann, R., Junker, U., Junker, K., et al. (2002). The serine proteinase thrombin promotes migration of human renal carcinoma cells by a PKA-dependent mechanism. Cancer Letters, 180(2), 183–190.
Tsopanoglou, N. E., & Maragoudakis, M. E. (2004). Role of thrombin in angiogenesis and tumor progression. Seminars in Thrombosis and Hemostasis, 30(1), 63–69.
Wojtukiewicz, M. Z., Tang, D. G., Nelson, K. K., Walz, D. A., Diglio, C. A., & Honn, K. V. (1992). Thrombin enhances tumor cell adhesive and metastatic properties via increased alpha IIb beta 3 expression on the cell surface. Thrombosis Research, 68(3), 233–245.
Zhu, Q., Luo, J., Wang, T., Ren, J., Hu, K., & Wu, G. (2012). The activation of protease-activated receptor 1 mediates proliferation and invasion of nasopharyngeal carcinoma cells. Oncology Reports, 28(1), 255–261.
Even-Ram, S. C., Maoz, M., et al. (2001). Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the alpha vbeta 5 integrin. Journal of Biological Chemistry, 276(14), 10952–10962.
Bai, S. Y., Xu, N., Chen, C., Song, Y. L., Hu, J., & Bai, C. X. (2015). Integrin αvβ5 as a biomarker for the assessment of nonsmall cell lung cancer metastasis and overall survival. Clinical Respiratory Journal, 9(4), 457–467.
Fujimoto, D., Hirono, Y., Goi, T., Katayama, K., & Yamaguchi, A. (2008). Prognostic value of protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) in gastric cancer. Anticancer Research, 28(2A), 847–854.
Boire, A., Covic, L., Agarwal, A., Jacques, S., Sherifi, S., & Kuliopulos, A. (2005). PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell, 120(3), 303–313.
Nierodzik, M., Plotkin, A., Kajumo, F., & Karpatkin, S. (1991). Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. Journal of Clinical Investigation, 87(1), 229–236.
Wojtukiewicz, M. Z., Tang, D. G., Ben-Josef, E., Renaud, C., Walz, D. A., & Honn, K. V. (1995). Solid tumor cells express functional “tethered ligand” thrombin receptor. Cancer Research, 55(3), 698–704.
Nierodzik, M. L., Klepfish, A., & Karpatkin, S. (1995). Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thrombosis and Haemostasis, 74(1), 282–290.
Huang, Y. Q., Li, J.-J., Hu, L., & Karpatkin, S. (2002). Thrombin induces the synthesis of VEGF and angiopoietin-2 (Ang-2). Blood, 99(5), 1646–1650.
Mohle, R., Green, D., Moore, M., Nachman, R., & Rafii, S. (1997). Constitutive production and thrombin-induced release of VEGF by human megakaryocytes and platelets. Proceeding of the National Academy of Sciences USA, 94(2), 663–668.
Li, J.-J., Huang, Y.-Q., Basch, R., & Karpatkin, S. (2001). Thrombin induces the release of angiopoietin-1 from platelets. Thrombosis and Haemostasis, 85(2), 204–206.
Belting, M., Dorrell, M. I., Sandgren, S., et al. (2004). Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nature Medicine, 10(5), 502–509.
Trivedi, V., Boire, A., Tchernychev, B., et al. (2009). Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell, 137(2), 332–343.
Sebastiano, M., Momi, S., Falcinelli, E., Bury, L., Hoylaerts, M. F., & Gresele, P. (2017). A novel mechanism regulating human platelet activation by MMP-2-mediated PAR1 biased signaling. Blood, 129(7), 883–895.
Jurasz, P., Sawicki, G., Duszyk, M., et al. (2001). Matrix metalloproteinase 2 in tumor cell-induced platelet aggregation: regulation by nitric oxide. Cancer Research, 61(1), 376–382.
Martin, C., Mahon, G., Klinger, M. B., et al. (2001). The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene, 20(16), 1953–1963.
Nierodzik, M. L., & Karpatkin, S. (2006). Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell, 10(5), 355–362.
Even-Ram, S., Uziely, B., Cohen, P., et al. (1998). Thrombin receptor overexpression in malignant and physiological invasion processes. Nature Medicine, 4(8), 909–914.
Bar-Shavit, R., Turm, H., Salah, Z., Maoz, M., Cohen, I., Weiss, E., et al. (2011). PAR1 plays a role in epithelial malignancies: transcriptional regulation and novel signaling pathway. International Union of Biochemistry and Molecular Biology Life, 63(6), 397–402.
Yin, Y. J., Salah, Z., Grisaru-Granovsky, S., et al. (2003). Human protease-activated receptor-1 expression in malignant epithelia: a role in invasiveness. Ateriosclerosis Thrombosis and Vascular Biology, 23(6), 940–944.
Yin, Y. J., Salah, Z., Grisaru-Granovsky, S., et al. (2003). Oncogenic transformation induces tumor angiogenesis: a role for PAR1 activation. Federation of American Societies for Experimental Biology Journal, 17(2), 163–174.
Zigler, M., Kamiya, T., Brantley, E. C., Villares, G. J., & Bar-Eli, M. (2011). PAR-1 and thrombin: the ties that bind the microenvironment to melanoma metastasis. Cancer Research, 71(21), 6561–6566.
Morris, D. R., Ding, Y., Ricks, T. K., Gullapalli, A., Wolfe, B. L., & Trejo, J. (2006). Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Research, 66(1), 307–314.
Tsopanoglou, N. E., & Maragoudakis, M. E. (2007). Inhibition of angiogenesis by small-molecule antagonists of protease-activated receptor-1. Seminars in Thrombosis and Hemostasis, 33(7), 680–687.
Zania, P., Kritikou, S., Flordellis, C. S., Maragoudakis, M. E., & Tsopanoglou, N. E. (2006). Blockade of angiogenesis by small molecule antagonists to protease-activated receptor-1: association with endothelial cell growth suppression and induction of apoptosis. Journal of Pharmacology and Experimental Therapy, 318(1), 246–254.
Gurbel, P. A., Bliden, K. P., Turner, S. E., et al. (2016). Cell-penetrating pepducin therapy targeting PAR1 in subjects with coronary artery disease. Arterioscleriosclerosis Thrombosis and Vascular Biology, 36(1), 189–197.
Yang, E., Boire, A., Agarwal, A., et al. (2009). Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Research, 69(15), 6223–6231.
Cisowski, J., O'Callaghan, K., Kuliopulos, A., et al. (2011). Targeting protease-activated receptor-1 with cell-penetrating pepducins in lung cancer. American Journal of Pathology, 179(1), 513–523.
Agarwal, A., Covic, L., Sevigny, L. M., Kaneider, N. C., Lazarides, K., Azabdaftari, G., et al. (2008). Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Molecular Cancer Therapeutics, 7(9), 2746–2757.
Justus, C. R., & Yang, L. V. (2015). GPR4 decreases B16F10 melanoma cell spreading and regulates focal adhesion dynamics through the G13/Rho signaling pathway. Experimental Cell Research, 334(1), 100–113.
Bian, D., Mahanivong, C., Yu, J., et al. (2006). The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene, 25(15), 2234–2244.
Lan, T., Wang, H., Zhang, Z., et al. (2017). Downregulation of β-arrestin 1 suppresses glioblastoma cell growth and glycolysis via inhibition of Src signaling. Experimental Cell Research, 4827(17), 30251–30253.
Duan, X., Kong, Z., Liu, Y., et al. (2015). β-Arrestin2 contributes to cell viability and proliferation via the down-regulation of FOXO1 in castration-resistant prostate cancer. Journal of Cellular Physiology, 230(10), 2371–2381.
Kotula, J. W., Sun, J., Li, M., et al. (2014). Targeted disruption of β-arrestin 2-mediated signaling pathways by aptamer chimeras leads to inhibition of leukemic cell growth. PloS One, 9(4), e93441.
Xu, P., Zuo, H., Chen, B., et al. (2017). Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Scientific Reports, 7, 42632.
Sarkar, S., Alam, M. A., Shaw, J., & Dasgupta, A. K. (2013). Drug delivery using platelet cancer cell interaction. Pharmacological Research, 30(11), 2785–2794.
Xu, P., Zuo, H., Zhou, R., et al. (2017). Doxorubicin-loaded platelets conjugated with anti-CD22 mAbs: a novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget. doi:10.18632/oncotarget.16871.
Li, J., Sharkey, C. C., Wun, B., Liesveld, J. L., & King, M. R. (2016). Genetic engineering of platelets to neutralize circulating tumor cells. Journal of Controlled Release, 228, 38–47.
Li, J., Ai, Y., Wang, L., et al. (2016). Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials, 76, 52–65.
Hu, Q., Quian, C., Sun, W., et al. (2016). Engineered nanoplatelets for enhanced treatment of multiple myeloma and thrombus. Advanced Materials, 28(43), 9573–9580.
Żmigrodzka, M., Guzera, M., Miśkiewicz, A., Jagielski, D., & Winnicka, A. (2016). The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumour Biology, 37(11), 14391–14401.
Amison, R., Page, C., & Pitchford, S. C. (2012). Pharmacological modulation of the inflammatory actions of platelets. Handbook of Experimental Pharmacology, 210, 447–468.
Carboni, E., Tschudi, K., Nam, J., Lu, X., & Ma, A. W. (2014). Particle margination and its implications on intravenous anticancer drug delivery. AAPS PharmSciTech, 15(3), 762–771.
Wang, C., et al. (2017). In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nature Biomedical Engineering, 1, 0011. doi:10.1038/s41551-016-0011.
Gresele, P., Momi, S., Pitchford, S. C., & Page, C. P. (2008). Platelets in respiratory disorders and inflammatory conditions. In P. Gresele, V. Fuster, J. A. Lòpez, C. P. Page, & J. Vermylen (Eds.), Platelets in hematologic and cardiovascular disorders, A clinical handbook (pp. 323–340). Cambridge, UK: Cambridge University Press.
Gresele, P., Falcinelli, E., & Momi, S. (2008). Potentiation and priming of platelet activation: a potential target for antiplatelet therapy. Trends in Pharmacological Sciences, 29(7), 352–360.
Menter, D. G., Davis, J. S., Tucker, S. C., et al. (2017). Platelets: “first responders” in cancer progression and metastasis. In P. Gresele, N. Kleiman, J. A. Lopez, & C. P. Page (Eds.), Platelets in thrombotic and non-thrombotic disorders (Vol. 2, pp. 1111–1132). Switzerland: Springer, Cham.
Ware, J., & Post, S. R. (2017). Platelets and inflammatory disorders of connective tissue. In P. Gresele, N. Kleiman, J. A. Lopez, & C. P. Page (Eds.), Platelet in thrombotic and non-thrombotic disorders (Vol. 2, pp. 1133–1137). Switzerland: Springer, Cham.
Momi, S., Pitchford, S. C., Gresle, P., & Page, C. P. (2017). Platelets and airway diseases. In P. Gresele, N. Kleiman, J. A. Lopez, & C. P. Page (Eds.), Platelet in thrombotic and non-thrombotic disorders (Vol. 2, pp. 1149–1168). Switzerland: Springer, Cham.
Andre, P. (2017). Targeting intraplatelet signaling pathways as potential antithrombotic strategy. In P. Gresele, N. Kleiman, J. A. Lopez, & C. P. Page (Eds.), Platelet in thrombotic and non-thrombotic disorders (Vol. 2, pp. 1341–1360). Switzerland: Springer, Cham.
Acknowledgments
The authors would like to thank Dr. S. Orsini for skilled editorial assistance. This work was supported in part by a grant to P.G. from H2020-FETOPEN-2014-CSA (CIRCLE, proposal # 665564).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest associated with this manuscript.
Rights and permissions
About this article
Cite this article
Gresele, P., Momi, S., Malvestiti, M. et al. Platelet-targeted pharmacologic treatments as anti-cancer therapy. Cancer Metastasis Rev 36, 331–355 (2017). https://doi.org/10.1007/s10555-017-9679-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-017-9679-8