Abstract
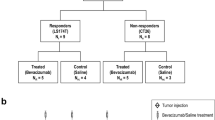
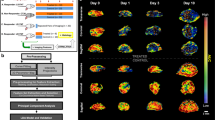
Due to spatial tumor heterogeneity and consecutive sampling errors, it is critically important to assess treatment response following antiangiogenic therapy in three dimensions as two-dimensional assessment has been shown to substantially over- and underestimate treatment response. In this study, we evaluated whether three-dimensional (3D) dynamic contrast-enhanced ultrasound (DCE-US) imaging allows assessing early changes in tumor perfusion following antiangiogenic treatment (bevacizumab administered at a dose of 10 mg/kg b.w.), and whether these changes could predict treatment response in colon cancer tumors that either are responsive (LS174T tumors) or none responsive (CT26) to the proposed treatment. Our results showed that the perfusion parameters of 3D DCE-US including peak enhancement (PE) and area under curve (AUC) significantly decreased by up to 69 and 77%, respectively, in LS174T tumors within 1 day after antiangiogenic treatment (P = 0.005), but not in CT26 tumors (P > 0.05). Similarly, the percentage area of neovasculature significantly decreased in treated versus control LS174T tumors (P < 0.001), but not in treated versus control CT26 tumors (P = 0.796). Early decrease in both PE and AUC by 45–50% was predictive of treatment response in 100% (95% CI 69.2, 100%) of responding tumors, and in 100% (95% CI 88.4, 100%) and 86.7% (95% CI 69.3, 96.2%), respectively, of nonresponding tumors. In conclusion, 3D DCE-US provides clinically relevant information on the variability of tumor response to antiangiogenic therapy and may be further developed as biomarker for predicting treatment outcomes.





Similar content being viewed by others
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29(6 Suppl 16):15–18. doi:10.1053/sonc.2002.37263
Shojaei F (2012) Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett 320(2):130–137. doi:10.1016/j.canlet.2012.03.008
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, Tonini G, Carlomagno C, Allegrini G, Chiara S, D’Amico M, Granetto C, Cazzaniga M, Boni L, Fontanini G, Falcone A (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16(13):1306–1315. doi:10.1016/S1470-2045(15)00122-9
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, Vieitez JM, Bouche O, Borg C, Steffens CC, Alonso-Orduna V, Schlichting C, Reyes-Rivera I, Bendahmane B, Andre T, Kubicka S, Investigators MLS (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14(1):29–37. doi:10.1016/S1470-2045(12)70477-1
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Hlatky L, Hahnfeldt P, Folkman J (2002) Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst 94(12):883–893
Marcus CD, Ladam-Marcus V, Cucu C, Bouche O, Lucas L, Hoeffel C (2009) Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol 72(3):217–238. doi:10.1016/j.critrevonc.2008.07.012
Hoyt K, Sorace A, Saini R (2012) Quantitative mapping of tumor vascularity using volumetric contrast-enhanced ultrasound. Investig Radiol 47(3):167–174. doi:10.1097/RLI.0b013e318234e6bc
Wang H, Hristov D, Qin J, Tian L, Willmann JK (2015) Three-dimensional dynamic contrast-enhanced US imaging for early antiangiogenic treatment assessment in a mouse colon cancer model. Radiology 277(2):424–434. doi:10.1148/radiol.2015142824
Wang H, Lutz AM, Hristov D, Tian L, Willmann JK (2017) Intra-animal comparison between three-dimensional molecularly targeted US and three-dimensional dynamic contrast-enhanced US for early antiangiogenic treatment assessment in colon cancer. Radiology 282(2):443–452. doi:10.1148/radiol.2016160032
Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, Harper SJ, Bates DO (2008) VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer 98(8):1366–1379. doi:10.1038/sj.bjc.6604308
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131(3):463–475. doi:10.1016/j.cell.2007.08.038
Xu L, Stevens J, Hilton MB, Seaman S, Conrads TP, Veenstra TD, Logsdon D, Morris H, Swing DA, Patel NL, Kalen J, Haines DC, Zudaire E, St Croix B (2014) COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med 6(242):242ra284. doi:10.1126/scitranslmed.3008455
Ren Y, Fleischmann D, Foygel K, Molvin L, Lutz AM, Koong AC, Jeffrey RB, Tian L, Willmann JK (2012) Antiangiogenic and radiation therapy: early effects on in vivo computed tomography perfusion parameters in human colon cancer xenografts in mice. Investig Radiol 47(1):25–32. doi:10.1097/RLI.0b013e31823a82f6
Heijmen L, Punt CJ, Ter Voert EG, de Geus-Oei LF, Heerschap A, Bussink J, Sweep CG, Zerbi V, Oyen WJ, Span PN, Boerman O, van Laarhoven HW (2013) Monitoring the effects of bevacizumab beyond progression in a murine colorectal cancer model: a functional imaging approach. Investig New Drugs 31(4):881–890. doi:10.1007/s10637-012-9920-9
Zhang HP, Shi QS, Li F, Liu L, Bai M, Gu JY, Wu Y, Du LF (2013) Regions of interest and parameters for the quantitative analysis of contrast-enhanced ultrasound to evaluate the anti-angiogenic effects of bevacizumab. Mol Med Rep 8(1):154–160. doi:10.3892/mmr.2013.1499
Heijmen L, Ter Voert EG, Punt CJ, Heerschap A, Oyen WJ, Bussink J, Sweep CG, Laverman P, Span PN, de Geus-Oei LF, Boerman OC, van Laarhoven HW (2014) Monitoring hypoxia and vasculature during bevacizumab treatment in a murine colorectal cancer model. Contrast Media Mol Imaging 9(3):237–245. doi:10.1002/cmmi.1564
Greis C (2011) Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc 49(1–4):137–149. doi:10.3233/CH-2011-1464
Schlosser J, Kirmizibayrak C, Shamdasani V, Metz S, Hristov D (2013) Automatic 3D ultrasound calibration for image guided therapy using intramodality image registration. Phys Med Biol 58(21):7481–7496. doi:10.1088/0031-9155/58/21/7481
Heckel F, Schwier M, Peitgen H-O (2009) Object oriented application development with MeVisLab and Python. GI Jahrestag 154:1338–1351
Strouthos C, Lampaskis M, Sboros V, McNeilly A, Averkiou M (2010) Indicator dilution models for the quantification of microvascular blood flow with bolus administration of ultrasound contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control 57(6):1296–1310. doi:10.1109/TUFFC.2010.1550
Quaia E (2011) Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol 21(3):604–615. doi:10.1007/s00330-010-1965-6
Wang JW, Zheng W, Liu JB, Chen Y, Cao LH, Luo RZ, Li AH, Zhou JH (2013) Assessment of early tumor response to cytotoxic chemotherapy with dynamic contrast-enhanced ultrasound in human breast cancer xenografts. PLoS ONE 8(3):e58274. doi:10.1371/journal.pone.0058274
Obermueller E, Vosseler S, Fusenig NE, Mueller MM (2004) Cooperative autocrine and paracrine functions of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the progression of skin carcinoma cells. Cancer Res 64(21):7801–7812. doi:10.1158/0008-5472.CAN-03-3301
Chen Y, Han F, Cao LH, Li C, Wang JW, Li Q, Zheng W, Guo ZX, Li AH, Zhou JH (2015) Dose-response relationship in cisplatin-treated breast cancer xenografts monitored with dynamic contrast-enhanced ultrasound. BMC Cancer 15:136. doi:10.1186/s12885-015-1170-8
Baetke SC, Rix A, Tranquart F, Schneider R, Lammers T, Kiessling F, Lederle W (2016) Squamous cell carcinoma xenografts: use of VEGFR2-targeted microbubbles for combined functional and molecular US to monitor antiangiogenic therapy effects. Radiology 278(2):430–440. doi:10.1148/radiol.2015142899
Zocco MA, Garcovich M, Lupascu A, Di Stasio E, Roccarina D, Annicchiarico BE, Riccardi L, Ainora ME, Ponziani F, Caracciolo G, Rapaccini GL, Landolfi R, Siciliano M, Pompili M, Gasbarrini A (2013) Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol 59(5):1014–1021. doi:10.1016/j.jhep.2013.06.011
Hoyt K, Umphrey H, Lockhart M, Robbin M, Forero-Torres A (2015) Ultrasound imaging of breast tumor perfusion and neovascular morphology. Ultrasound Med Biol 41(9):2292–2302. doi:10.1016/j.ultrasmedbio.2015.04.016
Williams R, Hudson JM, Lloyd BA, Sureshkumar AR, Lueck G, Milot L, Atri M, Bjarnason GA, Burns PN (2011) Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology 260(2):581–590. doi:10.1148/radiol.11101893
Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, Taieb S, Aziza R, Sarran A, Labbe-Devilliers C, Gallix B, Lucidarme O, Ptak Y, Rocher L, Caquot LM, Chagnon S, Marion D, Luciani A, Feutray S, Uzan-Augui J, Coiffier B, Benastou B, Koscielny S (2014) Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Investig Radiol 49(12):794–800. doi:10.1097/RLI.0000000000000085
Wang H, Kaneko OF, Tian L, Hristov D, Willmann JK (2015) Three-dimensional ultrasound molecular imaging of angiogenesis in colon cancer using a clinical matrix array ultrasound transducer. Investig Radiol 50(5):322–329. doi:10.1097/RLI.0000000000000128
Zhou J, Wang H, Zhang H, Lutz AM, Tian L, Hristov D, Willmann JK (2016) VEGFR2-targeted three-dimensional ultrasound imaging can predict responses to antiangiogenic therapy in preclinical models of colon cancer. Cancer Res 76(14):4081–4089. doi:10.1158/0008-5472.CAN-15-3271
Wang Y, Dong L, Bi Q, Ge X, Zhang X, Wu D, Fu J, Zhang C, Wang C, Li S (2011) Beyond antiangiogenesis: intratumorally injected bevacizumab plays a cisplatin-sensitizing role in squamous cell carcinomas in mice. Chemotherapy 57(3):244–252. doi:10.1159/000326485
Acknowledgements
We would like to thank Philips for providing the EPIQ7 US system. We would also like to thank the China Scholarship Council and Program for New Century Excellent Talents in University for providing funding for Dr. Jianhua Zhou to study abroad at Stanford University.
Funding
This research was supported by the NIH R01 CA155289 Grant (JKW) and NIH R01 CA195443 (JKW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, J., Zhang, H., Wang, H. et al. Early prediction of tumor response to bevacizumab treatment in murine colon cancer models using three-dimensional dynamic contrast-enhanced ultrasound imaging. Angiogenesis 20, 547–555 (2017). https://doi.org/10.1007/s10456-017-9566-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-017-9566-5