How the Benzene Tree Polluted the World
The organic compounds that enabled industrialization have unintended, long-lasting consequences for the planet’s life.
Updated at 11:52 a.m. ET on November 4, 2020.
Deep in the Mariana Trench, at depths lower than the Rockies are high, rests a tin of reduced-sodium Spam.
NOAA scientists caught sight of it last year near the mouth of the Mariana’s Sirena Deep. It isn’t an isolated incursion, but it was nevertheless startling, the sight of those timeless golden letters bright against the deep ocean bottom.
Shortly after came news from another team of scientists who had found in the Mariana an innovation less familiar than shelf-stable meat, but far more significant. In the bodies of deep-dwelling creatures were found traces of industrial chemicals responsible for the rise of modern America—polychlorinated biphenyls.
PCBs had been detected in Hirondellea gigas, tiny shrimp-like amphipods scooped up by deepwater trawlers. Results from the expedition, led by Newcastle University’s hadal-zone expert Alan Jamieson, were preliminarily released last year and then published in February.
PCBs have been found the world over—from the bed of the Hudson River to the fat of polar bears roaming the high Arctic—but never before in the creatures of the extreme deep, a bioregion about which science knows relatively little.
How PCBs reached the Mariana is still under investigation. Jamieson and colleagues speculated on multiple, regional sources. A nearby military base. The industrial corridors along the Asian coastline. And the Great Pacific Garbage Patch, where PCBs glom onto plastic particles caught in the current. Over time, the plastic degrades and descends into the depths, ferrying PCBs with them.
But the true origin of PCBs lies in another time and place, in Depression-era Alabama, and before that, 19th-century Germany at the pinnacle of German chemistry.
* * *
PCB production began in late 1929 in a factory east of Birmingham. The same era that gave us New York’s Chrysler Building, The Little Engine That Could, and eventually Spam brought mass-made PCBs to market.
General Electric and Westinghouse were early adopters. Both firms formulated PCBs into dielectric fluids, the insulating liquids added to capacitors and other electrical components to keep them cool and to prevent fires. With PCBs’ aid, the electric grid spread from the industrialized north into the rural regions of the Deep South and the American West. By mid-century, PCBs had a bird’s-eye view of any block in America with a utility pole and PCB-bathed transformer.
Soon PCBs were added to paints, caulks, plastics, even floor finishes and dish detergents. They were branded, and assigned names like Aroclor. That commercial products contained PCBs was never advertised, explained Ellen Griffith Spears, who wrote the definitive book on PCBs’ genesis.
PCBs slipped into the world, becoming ubiquitous while remaining anonymous. Until the mid-1960s, when the Danish-born scientist Sören Jensen detected PCBs in the bodies of pike taken from the waters off Sweden.
In the wake of Rachel Carson’s Silent Spring, published in 1962, Jensen had been dispatched to look for DDT, one of the post-WWII pesticides whose increasing use Carson’s book had questioned. Jensen found DDT. But his data also signaled the presence of unexpected, yet chemically similar, contaminants. It took two years to determine the “ghosts” in his data were PCBs.
After that, everywhere Jensen thought to look, he found their fingerprints: in the feathers of archived white-tailed eagles, and in hair plucked from his own head, and others sampled from his wife and infant daughter. His conclusions, published in 1966, instigated a global investigation into the fate and toxicity of PCBs, research now carried forward (and into the deep oceans) by Jamieson and colleagues.
Today, PCBs are well-characterized pollutants—toxic, extremely persistent, and pervasive. All 209 variations of PCBs are known carcinogens. PCBs can alter liver function, and they can interfere with how humans reproduce, develop, think, and mount an immune response. Based on their cancer-causing potential alone, Congress voted to end American production in 1976 by attaching an amendment to the Toxic-Substances Control Act (TSCA).
“ToSCA,” as the law was called, gave the fledgling Environmental Protection Agency, created six years earlier, the authority to regulate industrial chemicals. PCBs were the only class of chemicals called out by TSCA; about 60,000 others were grandfathered, meaning their use was never questioned.
Another three years passed before Congress’s limits on PCB production took effect. Four decades later, though banned, PCBs live on, including in tiny amphipods swimming in some of the deepest waters of Earth’s biggest ocean.
* * *
PCBs, now endemic to environments everywhere, belong to a class of chemicals called (depending on the era) halocarbons, organohalogens, or halogenated organic compounds.
Organic, in this instance, refers not to foods raised without chemicals but to compounds made with carbon. Halo- (or halogen) signifies the presence of one or more of the halogen elements, the most familiar being chlorine, bromine, and fluorine. From these starting materials, chemists can make an array of compounds. But so can nature. To date, there are more than 5,000 so-called biogenic or naturally-occurring organohalogens.
Nature’s versions are forged in volcanoes or near deep-sea vents where temperatures run high, and chlorine and bromine are abundant. Organisms in all kingdoms can make halocarbons, though in minute quantities and typically for highly specialized purposes such as self-defense or signaling mates.
There are many pathways to making such complex molecules. The Iowa atmospheric chemist Scott Spak keeps a running tally of the “recipes” that might yield a PCB. While there are no known analogs in nature, one does get to wondering whether nature—at some point, somewhere on the planet or deep in the cosmos—could have served up the right mix of raw materials, in the right order and under the right conditions to allow for PCBs’ spontaneous formation. It is conceivable, Spak concedes, though purely hypothetical. Such a discovery, should it even occur, wouldn’t explain PCBs’ global dispersion, nor absolve what humans made with impunity. But it does hint at the complexity of Earth’s chemistry, and the humility with which we still endeavor to understand it.
Into intricate ecological and biological systems human industry introduced PCBs in extraordinary volumes, and in evolutionary terms, rapidly—over the span of three or four human generations, said Spak. But the problem isn’t so much that PCBs are “unnatural,” though one could make that argument. It is that they are molecules nature recognized, familiar enough to be folded into its systems and to confuse them.
Human biology has not adapted to their presence. Species far older than us, microbes mostly, have evolved over millennia to coexist with, and even to synthesize and break down, specific types of biogenic halocarbons. Some strains of bacteria are capable of disassembling PCBs. Other species, such as the Atlantic tomcod, bottom-feeders in the Hudson, have even developed a genetic variation that allows for survival in PCB-polluted waters, though their livers are also loaded with the chemicals.
But for humans, research tracking the health effects of industrial PCB exposures, Spak said, is tantamount to watching evolutionary consequences playing out in real time.
* * *
While sunlight (and some microorganisms) break down PCBs over time, they can be stunningly stable when stored in sediment, glaciers and other so-called “sinks” like the deep ocean. And because PCBs are lipophilic (or fat-loving), they can also accumulate in the fatty tissue of marine life, and in the bodies of mammals like us. Depending on the total load, some measure of PCBs can last a lifetime, or pass between generations through cord blood or milk.
The chemistry that enabled humankind to engineer elements into such durable molecules and enduring pollutants dates to the early 19th century, to a time when the natural world was the chemist’s muse. As chemistry industrialized, chemists were drawn by profit, and later, into geopolitics. In time, chemistry became a tool for nature’s mastery, and—both knowingly and inadvertently—an engine for its alteration.
At the center of this transformation is an elegant molecule called benzene. It is the same carbon-rich compound that lends gas stations their curiously evocative aroma, and PCBs their structural integrity.
A molecule of benzene is comprised of six carbon and six hydrogen atoms. Ask a chemist what benzene looks like, and she will draw a hexagon. It’s a schematic of how benzene’s six carbons circle themselves, as if linked hand in hand, into a ring. The benzene ring.
Benzene rings are also abundant in nature. They were present in Earth’s prebiotic soup, and they float in deep space. Many human hormones have benzene rings. And so do many human-engineered molecules.
Chemical engineers approach benzene as a building block from which to make thousands of useful products, including aspirin, the plastic lid to your takeaway coffee, and the Legos children leave strewn across floors the world over. Though, it should be noted that benzene is rarely made intentionally. Benzene is incidental to other industrial processes, such as refining oil into gasoline, processing coal into coke, or in the making of ethylene, another widely used chemical building block. Which makes benzene an industrial by-product and also a common industrial pollutant, especially following industrial accidents, as happened after Hurricane Harvey struck the plants along Texas’s Gulf coast.
All of which means the benzene ring is something of a paradox. When incorporated into certain molecules, it is essential to life. But in other configurations, slight tweaks in the composition and arrangement of atoms render benzene part of a toxic, possibly carcinogenic molecule. And when on its own, there is ample evidence that benzene causes cancer.
* * *
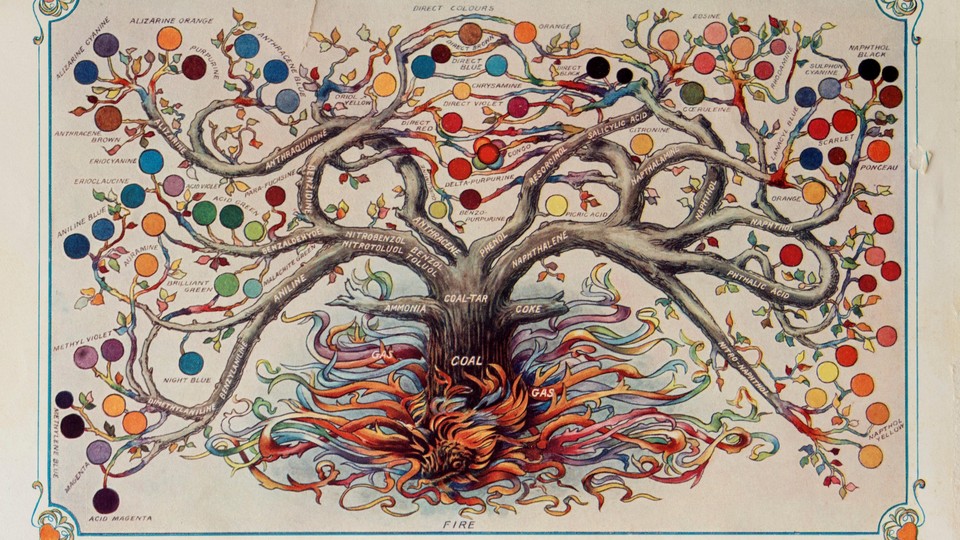
Until the middle of the 19th century, building on the benzene ring wasn’t possible because, though benzene had been isolated, its structure still eluded chemists. So significant was the “discovery” of benzene’s ring of carbons that in 1890, on the occasion of its 25th anniversary, the German Chemical Society threw a party—the Benzolfest! It was “a festival of magnificence perhaps unparalleled in the history of science,” wrote one commentator.
It was held at Berlin’s City Hall in Germany. The emperor was invited. Dignitaries came. All of the era’s most preeminent chemists gathered in their finest attire. August Hofmann, a bearded and towering figure in the field of organic chemistry, waxed poetic about benzene and the chemist, Friedrich August Kekulé, who had “pluck[ed] the heart out of its mystery.”*
The benzene tree, as he called it, was “thronged with blossoms,” its branches heavy with fruit. It was “a blaze of color,” and it gave off “an almost overwhelming fragrance,” a fitting metaphor given benzene’s signature scent.

To Hofmann, the benzene tree was a “giant.” It soared “into the clouds to where the eye cannot yet follow it.” Up the tree scaled “no dearth of industrious workers,” all “busily striving to collect the harvest,” he had said, referring to how entrepreneurial chemists were converting benzene chemistry into industrial products. “Keen climbers have already clambered up to the third or fourth branch,” Hofmann continued, some chemists “working at a dizzy[ing] height.”
With new insight into molecular configurations, chemists, starting around 1865, could better anticipate the steps required to synthesize new molecules. With the benzene ring, “the number of organic compounds all at once,” Hofmann had noted, “increased to infinity.”
Among those in the tree’s farthest reaches was Gustav Schultz, in attendance that night. In 1881, Schutlz, with the chemist Hermann Schmidt, had described the synthesis of a PCB. The pair published the new molecule in a premier German-language journal. And then, like DDT, also achieved during this era, PCBs were left to languish for decades on dust-cloaked shelves of obscure chemical libraries. Their discovery advanced chemical knowledge but was otherwise of little practical value.
* * *
Hofmann was born into the fast-changing world of 19th-century Europe. He grew up in parallel with organic chemistry, and ascended to its highest ranks. Unlike inorganic chemists’ fascination with Earth’s metals and minerals, the first organic chemists studied molecules from living organisms, which were principally comprised of carbon.
Benzene was first isolated from the by-products left after making “portable gas,” which was rendered from fish or whale blubber to fuel lamps. In time, chemists learned to derive benzene and other carbon-rich materials from coal tar instead, a project central to Hofmann’s work and that of his student, Charles Mansfield.
A lump of coal, while not alive, is evidence of life once lived. It is the long-sequestered remnants of ancient flora, from an epoch when trees could grow as tall, or taller, than the one Hofmann had conjured. Coal tar, though, is what remains after human extraction and use. Wherever cities gasified coal to light 19th-century streetlamps, or converted coal into hotter-burning coke to smelt metals, coal tar piled up as waste. Hofmann, curious of its composition, set out to characterize it.
But when organic chemistry adopted coal tar as its primary feedstock, it wedded itself to the residues of industrialization. And so the field became one step removed from the thrum of life that had first inspired it.
By the Benzolfest, organic chemistry was high technology, and the German Empire its Silicon Valley. The field was “the earliest pure science to have a massive impact on technology and on a national economy,” noted the historian Alan Rocke. Germany’s prowess in chemical manufacturing would embolden its nationalistic ambitions. But the war such nationalism inspired would seed the downfall of the German chemical empire, and the rise of a new kind of chemical industry on American soil.
* * *
The original chemical products plucked from the benzene tree had a very specific application: They were textile dyes, what turns humdrum fabrics into every hue imaginable. The first colorfast dye synthesized from coal tar had been an accident. A failed attempt by one of Hofmann’s students to make quinine—a hard-to-source malarial drug derived from the South American fever-bark tree—led to the discovery in 1856 of a dye that could permanently stain silk a rich, red-purple mauve.
Hofmann was based in England, and it was his English student William Perkin who happened into the colorant. Perkin left the lab against Hofmann’s counsel to build a dye-works instead. His factory marked the first attempt to do organic chemistry at scale, wrote Simon Garfield in his history of this world-changing invention. Eventually, after multiple failed attempts, Perkin developed the multistepped process, and proceeded to the equally difficult task of convincing an established, but reluctant, textile industry to adopt his industrial dye.
In time, Garfield noted, a new class of chemists engineered the radiant color palette of Victorian fashion from the dregs of Europe’s Industrial Revolution. Dye-works sprouted across Europe, clustering along Germany and Switzerland’s swift rivers. In time, these ran foul—discolored and odorous. Neighbors complained. Researchers took note. Governments acted, but the coal-tar industry bloomed all the same.
By the close of the 19th century, German dyestuffs dominated the world market, though the first effects of acute exposures were already evident among the earliest generations of dye workers. By 1897, the term chloracne appeared in the German literature to describe a condition unique to chemical workers, “an industrial leprosy,” where the skin is pocked with painful, disfiguring lesions. Not long after, medicine documented new “aniline (dye) tumors” and “dye-workers cancer.” In time, chemists realized benzene, too, was more hazardous in factory quantities than in those used in a laboratory.
And yet, coal-tar chemistry flourished, spreading in multiple directions at once. Chemical innovation led to new dyes, and also to new drugs like aspirin, a synthetic version of a molecule once sourced from willow bark. New classes of pharmaceuticals followed. In time, coal-tar drugs transformed health care.
But coal-tar drugs and coal-tar chemicals thereafter diverged, at least in the popular imagination and in the eyes of states trying to manage these burgeoning technologies. To this day, and despite recent reforms to U.S. policy, drugs and industrial chemicals fall under the purview of separate laws administered by separate agencies. And they are held to separate standards of safety. Forgotten to history are their common origins and chemical ancestry.
Less than a century after the Benzolfest, scientists, led by Theo Colborn, synthesized the growing body of ecological research into a disquieting discovery. At trace levels of exposure, levels lower than workers’ acute exposures and equivalent to dosed drugs, many of these new classes of organic chemicals (including PCBs) could mimic, block, or disrupt the work of hormones, the biochemical signals that coordinate multicellular life.
* * *
Though Perkin missed the mark, making mauve instead, it was no accident that an English chemist sought a synthetic route to quinine. Malarial drugs were essential to colonial expansion into the tropics, one example of how organic chemicals are influenced by prevailing geopolitics and pressed to do the work of empire.
By the second decade of the 20th century, organic chemicals were moved to the front lines of global conflict. Mustard gas, picric acid, and TNT—all organic molecules—made for unimaginably destructive weapons. After Versailles, even peacetime users of organic chemicals were hailed as patriots and power brokers. Germany paid reparations with dyes, while the United States seized German chemical patents and industrial-plant designs as the spoils of war.
Before the Great War, few U.S. firms had ventured into organic chemicals. Only after fighting began, and the British embargo blocked import of German chemicals, did American companies leap into the unknown of organic-chemicals production.
“Americans keenly felt their dependence on German chemicals,” wrote the industrial historian Kathryn Steen. Mastering how to make them domestically was motivated “partly because of the[se] shortages,” she added, but “primarily because of what [the chemicals] represented to Americans—the seemingly inferior industrial and scientific abilities relative to the enemy and rival.”
After the war, growing the nation’s capacity to manufacture chemicals became a national project. The founder of Hooker Chemical (later responsible for Love Canal, the nation’s first Superfund site) argued this point with regard to chlorine (a gruesome war gas). The same case was made for coal-tar chemicals in 1917 by John F. Queeny, the founder of Monsanto Chemical—the company that two decades later would take PCBs global. Factories that made drugs and dyes from coal, Queeny argued, could easily make the materials required by modern warfare. Ramp up peacetime production of coal-tar chemicals and a fleet of war-ready factories would be lying in wait. It would mark “the declaration of American chemical independence,” Queeny said.

For the general public, though, the new coal-tar chemicals were a harder sell.
Coal-tar chemicals smacked of artifice and, in their use as chemical weapons, seemed abhorrent, not the patriots industrialists had painted them to be. Chemistry had a postwar public-relations problem.
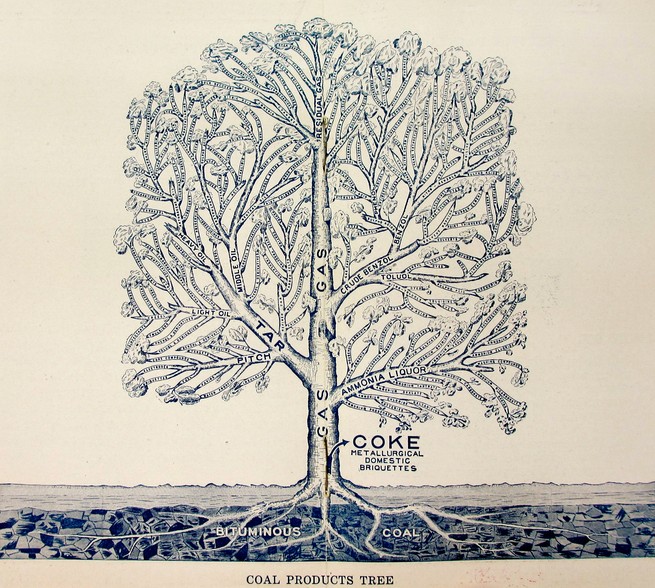
In the same editorial where Queeny equated national security with a robust chemical industry, he also pictured coal-tar chemistry as a thickly crowned tree. Like those that chart ancestry, Queeny’s tree arranged chemical products by family and parentage.
Semet-Solvay widely distributed a similar image. Their “coal products tree” rooted the company’s offerings in the nation’s bituminous coal beds. Along boughs of ammonia, tar, and benzene bloomed hundreds of products—Saccharin! Explosives! Mothballs! Perfume!—all promising mastery over nature’s unyielding cycles, its wild swarms and infestations, its off-putting odors and inconvenient secretions.
The collective conscience still carried residues of the real and symbolic horrors of chemical warfare, even at the height of the roaring ’20s. Chemistry had a spectral presence, and it was evident in popular sentiment and the era’s literature. All Quiet on the Western Front, A Farewell to Arms, and Goodbye to All That were postmortems on chemical warfare. All three were published (or translated) in 1929, the same year PCB production began far from the battlefields of Western Europe in Anniston, Alabama, on the lush, southern limits of Appalachia’s rolling hills.
Anniston was a planned, industrial utopia. But in reality, it was a segregated southern city populated by pig-iron and pipe foundries. PCBs were first made by Anniston’s Federal Phosphorus Company, in a plant that belonged to the son of a Confederate solider, a charismatic entrepreneur and a champion of the South’s revival.
Theodore Swann had gotten his start selling locomotives and later electricity. During World War I, he managed a munitions plant in Anniston, but then founded a factory to refine manganese for the steel industry, and when that failed, phosphorous, which is how Swann finally came into his fortune. His electric furnaces captured a pure form of phosphoric acid, which was sold to make fertilizer, detergent, baking soda and even soda pop.
Swann wasn’t a chemist, but a “boom man in a boomtown,” as one biographer put it, always looking for the next thing. And in the mid-1920s, what was on the rise were cars, American crude, and by extension organic chemicals. The success of these ascendant industries depended on better chemical technologies to make better fuels and better car parts. And Swann had seen it coming.
In the mid-1920s, at the invitation of an oil company, Indian Refining (later known as Texaco), he agreed to manufacture a benzene-based chemical called diphenyl (or biphenyl, in today’s parlance). Indian Refining needed a regular supply of diphenyl, a heat transfer fluid, to improve its oil-refining process. Except only one company made diphenyl, Eastman Kodak, and it was only available in small batches at the prohibitive price of $40 per pound.
The structure of diphenyl was simple: just two fused rings of benzene. But it required an entirely different chemistry, and experience and expertise that were in short supply at the time.
As Spears described in Baptized in PCBs, Swann assigned a team to the difficult (and hazardous) task, and issued them a deadline. Development proceeded by trial and error, with error sometimes resulting in explosions, including one that took off a wall and showered Swann’s men with fire, glass, benzene, and other shrapnel.
They eventually mastered diphenyl, a “magic fluid” that brought the nation to the brink of a new chemical age. Not long after, Swann’s chemists scaled another process to add chlorine onto the diphenyl backbone, creating a molecule so stable, it would travel the globe and accumulate in places surely unfathomable to Swann. Swann had financed the conversion of benzene into biphenyl, and now biphenyl into PCBs. The Anniston Works would soon produce 3,000 pounds of PCBs per day.
Production levels climbed higher by the year, as PCBs were put to the project of nation-building. They erected the bases and surveillance equipment that protected the new world order, while at home, PCBs were built into the schools, offices, and factories constructed to accommodate the postwar boom. PCBs would transform U.S. industry in a matter of decades and global ecology before the century was out.
* * *
In the United States, the first batches of PCBs, made at the close of the 1920s, likely left Anniston for Pittsburgh, home of H.H. Robertson Company. Robertson made prefabricated metal siding and roofing from which to erect factories, smelters, refineries and chemical works. The company, like Indian Refining before them, had asked for technical assistance. They needed a new protective coating for their metal sheeting, one that could prevent erosion and the spread of fire better than Halowaxes, or chlorinated naphthalene, one of the earliest classes of industrial halocarbons produced in the United States.
PCBs were unusually durable and durably useful. They were heat-resistant, non-conductive, and excellent as a weatherproof and fireproof coating. And because they were a value-added waste product, PCBs were economically viable.
Flame-resistant factory parts may seem like an insignificant side note, but Robertson’s Protected Metals, later called Galbestos, were instrumental in growing the nation’s manufacturing sector in size and scale. Factories were changing. Small, clustered, brick structures gave way to multistory, steel Goliaths. And these needed to withstand the elements, and to contain the fire and explosion risks that went along with the 20th century’s new methods for transforming nature’s resources into the mass-made materials of modern societies.
Four days after the stock-market crash of 1929, Swann’s firm filed a patent for PCB-laced transformer oils. Westinghouse and GE, like Robertson, would soon find PCBs indispensable, wrote Spears.
But despite these early successes, problems were mounting in Anniston. Within the first years of PCB production, those handling PCBs developed the same chloracne and other debilitating symptoms as dye workers a generation earlier. Three workers at Halowax who handled PCBs died from acute yellow atrophy of the liver (extreme jaundice), fatalities that were studied, but ultimately cast aside by company officials.
And though Swann’s company had weathered the worst years of the Depression, it wouldn’t survive the decade. Roosevelt’s National Recovery Administration had issued economic directives that, as Swann put it, leveled southern industry like Sherman’s March to the Sea. And he had been a profligate spender—living high in the Birmingham hills. But with shifting costs, and loans coming due, Swann went under. His rise to fortune and influence had been fast and somewhat famed. (He had even been profiled in Forbes.) His decline was equally precipitous. Swann sunk into debt, losing his mansion, and all claims to his many factories and technologies.
Queeny’s coal-tar drug company, Monsanto, was also flush from lucrative contracts with Coca-Cola, who bought their caffeine, vanillin, and saccharin. Monsanto took over PCB production in 1935, securing government contracts, growing sales through World War II, and shielding all along the suite of chemicals from regulation until the mid-1970s.
Chemical production in these intervening years soared, owning to the fact that U.S. firms switched their substrate from coal tar to the by-products of the new, advanced crude refineries. In the 1950s, Socony-Vacuum (later ExxonMobil) published the petroleum tree, emblematic of the flowering of American petrochemistry, at the time a “uniquely American phenomenon,” as industrialist Peter Spitz put it.
Two years later, in 1959, Goodrich-Gulf published the rubber tree, as symbolic to this era as Hofmann’s tree had been in his time. Advances in chemical engineering had obviated the need for natural latex, making rubber from oil rather than rubber trees. Drugs, sweeteners, flavorings, fertilizers, fabrics, and furnishings now all had synthetic equivalents. Chemistry, it seemed, had freed humanity from nature altogether.
When chemical trees finally disappeared from popular culture, what was lost was any connection of organic chemicals to their fossil-fuel roots, and of greater significance, to their molecular basis in carbon and the chemistry of life.
* * *
If you bore into the fat-rich bark of a thick-trunked tree, you’ll likely find PCBs, same as you’d find in deep-ocean amphipods, which makes trees like shrimp, and shrimp like us. PCBs are thought to be present in detectable levels in every person on the planet. Though everywhere, the implications are distributed unevenly. PCBs can concentrate, creating hot spots, including in Anniston and other factory towns and regions of the Arctic and subarctic, with significant implications for the indigenous communities living there.
Despite national and international curbs on their production, PCBs now congregate in the deep ocean, raising new concerns. In some areas of the Mariana, PCB levels registered 50 times higher than those found in crabs living in surface waters near heavy industry in China.
By the time the international community stepped in to end global PCB production, well over 1 million metric tons (about 3 billion pounds) had been manufactured worldwide. The 2001 UN Stockholm Convention on Persistent Organic Pollutants that resulted from these negotiations, but which the United States has yet to ratify, initially named DDT, PCBs, and 10 other chemicals (or classes of chemicals), all based on benzene.
“The challenge moving forward is to determine the physiological consequences of such contamination and understand knock-on effects on ecosystem function,” Jamieson and his colleagues concluded in the pages of Nature Ecology and Evolution. Except human activity may be altering the chemistry of the deep before we have had the chance to document it.
The problem is not limited to PCBs, but extends to the larger family of organohalogens to which they belong. In the early 1970s, PCBs were replaced by other organohalogens, one being polybrominated biphenyls, or PBBs, molecules similar to PCBs, but made with bromine instead of chlorine. Soon after their introduction, PBBs got into cattle feed, poisoning the food supply and the people of Michigan, and saddling the state with a long-lived legacy. PBDEs, polybrominated diphenyl ethers, followed as yet another alternative. These were used as flame retardants for two decades before a subsequent generation of Swedish scientists charted rising levels in breast milk.
Besides PCBs, amphipods living in the Mariana also harbor PBDEs, though the most prevalent commercial mixtures have been phased out of U.S. production and named to the UN Stockholm Convention as well.
The deep now archives nearly a century of chemical innovation, and documents the rise and fall of chemical classes, which industry develops and retracts in waves without seeming to absorb the larger lesson.
* * *
There are those who want a bright line to divide what’s natural from what isn’t as a means to make clear what’s safe. But with their origins in Earth’s deep carbon, and their enduring presence in life forms everywhere, such distinctions are murky at best. And yet, PCBs are part of a post-natural state in which industrial chemistry and ecology have become one and the same.
“Nature,” the organic chemist Pat Costner reminded me, “is a chemist, too” and the world its roiling, bubbling, reactive laboratory. Science has only begun to grapple with the complexity of our overlapping chemistries. Costner trained in organic chemistry at the peak of the chemical age. She took her first job as a bench chemist with Shell Oil in the 1960s, though she didn’t stay long. She turned her attention to organic pollution, particularly the chemical by-products industry never intended to make but released into nature anyway.
Her specialty became the family of benzene-based compounds called dioxins, perhaps the most poisonous products of the benzene tree. Some PCBs, she reminded me, are dioxin-like in their toxicity profile. And like dioxins, PCBs can also be inadvertent by-products, made incidentally during the manufacture of titanium dioxide, for example, or a shade of yellow organic inks and dyes reminiscent of the letters that spell out Spam. Try though we may, control over chemistries this complex is something of a chimera.
“We have been so clever at learning to play with atoms and molecules without ever thinking about what they do once they are out,” she told me. “Put a complex molecule into the environment,” said Costner, “and it is going to undergo any number of transitions in hard-to-predict ways.”
The same is true when human-made molecules interact with the exquisite biochemistry of our bodies.
As the biologist Sandra Steingraber explains, the organs of the human system are designed to “shuttle around, break apart, recycle, and reconstruct carbon-containing molecules,” work orchestrated by enzymes and hormones. If carbon molecules come with add-ons like chlorine and bromine, the chemical makeup can influence whether the body stores the molecules, metabolizes them, renders them benign, or makes them inherently more dangerous.
But regulating organic chemicals for their biological activity has been political and controversial because carbon is not just the basis of our biology, but also deeply embedded in our economy.
One hundred and fifty years ago, when Hofmann and his contemporaries gathered to celebrate benzene, humanity’s collective relationship to carbon and nature had already begun to shift. Chemistry, which once mimicked nature’s molecules, had begun to manipulate them. All the more striking, then, is Hofmann’s fantastical tree. Just two years before his death, he rooted chemistry to the Earth. However high chemists might climb, however much industry might harvest, chemistry was grounded in the laws of nature. What goes around comes around. Nature travels in cycles.
But those in attendance that cold March eve, a decade before the new century dawned, could not have known that the branch of chemistry they honored—founded to study living matter!—would spawn an industry so prolific as to irreparably alter the chemistry of life itself.
The chemical innovations of Hofmann’s era would get swept into global conflict—and the American impulse toward maximum and unfettered production—casting PCBs and other fruits of the benzene tree far and wide, into the ocean deep and possibly the depths of time.
*This article originally misspelled August Hofmann's name.