Scientists have tried editing a gene inside the body for the first time, in a bold attempt to tackle an incurable a disease by permanently changing a patient’s DNA.
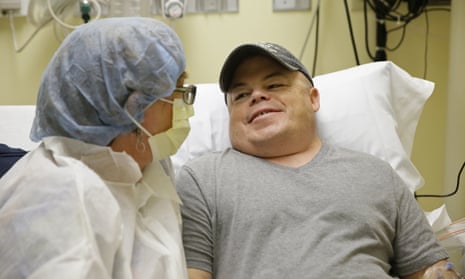
On Monday in California, 44-year-old Brian Madeux intravenously received billions of copies of a corrective gene and a genetic tool to cut his DNA in a precise spot.
“It’s kind of humbling to be the first to test this,” said Madeux, who has a metabolic disease called Hunter syndrome. “I’m willing to take that risk. Hopefully it will help me and other people.”
Signs of whether it is working may come in a month; tests will confirm in three months.
If successful, the new technique could give a major boost to the fledgling field of gene therapy. Scientists have edited people’s genes before, altering cells in the lab that are then returned to patients. There also are gene therapies that do not involve editing DNA.
But these methods can only be used for a few types of diseases. Some give results that may not last. Some others supply a new gene like a spare part, but can’t control where it inserts in the DNA, possibly causing a new problem, such as cancer.
This time, the genetic tinkering is happening in a precise way inside the body – like sending a miniature surgeon along to place the new gene in exactly the right location.
“We cut your DNA, open it up, insert a gene, stitch it back up. Invisible mending,” said Dr Sandy Macrae, president of Sangamo Therapeutics, the California company testing the therapy for two metabolic diseases and haemophilia. “It becomes part of your DNA and is there for the rest of your life.”
That also means there is no way to erase any mistakes the editing might cause.
The risks can’t be fully known, but because these are incurable diseases the studies should move forward, said one independent expert, Dr Eric Topol of the Scripps Translational Science Institute in San Diego.
Protections are in place to help ensure safety, and animal tests were very encouraging, according to Dr Howard Kaufman, a Boston scientist on the National Institutes of Health panel that approved the studies.
He said gene editing’s promise is too great to ignore. “So far there’s been no evidence that this is going to be dangerous,” he said. “Now is not the time to get scared.”
Fewer than 10,000 people worldwide have these metabolic diseases, partly because many die very young. Those with Madeux’s condition lack a gene that makes an enzyme that breaks down certain carbohydrates. These build up in cells and cause havoc throughout the body.
Patients may have frequent colds and ear infections, distorted facial features, hearing loss, heart problems, breathing trouble, skin and eye problems, bone and joint flaws, bowel issues and brain and cognitive problems.
“Many are in wheelchairs … dependent on their parents until they die,” said Dr. Chester Whitley, a University of Minnesota genetics expert who plans to enrol patients in the studies.
Currently, weekly doses of the missing enzyme can ease some symptoms, but cost $100,000 to $400,000 a year and do not prevent brain damage.
Madeux, who now lives near Phoenix, Arizona, is engaged to a nurse, Marcie Humphrey, who he met 15 years ago in a study that tested the enzyme therapy at UCSF Benioff Children’s Hospital Oakland, where the gene editing experiment also took place.
He has had 26 operations for hernias, bunions, bones pinching his spinal column, and ear, eye and gall bladder problems.
“It seems like I had a surgery every other year of my life,” he said. Last year he nearly died from an attack of bronchitis and pneumonia. The disease had warped his airway: “I was drowning in my secretions, I couldn’t cough it out.”
Gene editing will not fix damage he’s already suffered, but he hopes it will end the need for weekly enzyme treatments.
Initial studies will involve up to 30 adults to test safety, but the ultimate goal is to treat children very young, before much damage occurs.
The gene-editing tool Crispr-Cas9 has had a lot of recent attention, but this study used a different tool called zinc finger nucleases. They work like molecular scissors that seek and cut a specific piece of DNA.
The therapy has three parts: the new gene and two zinc finger proteins. DNA instructions for each part are placed in a virus that has been altered to not cause infection but instead to ferry them into cells. Billions of copies of these are given to the patient intravenously.
They travel to the liver, where cells use the instructions to make the zinc fingers and prepare the corrective gene. The fingers cut the DNA, allowing the new gene to slip in. The new gene then directs the cell to make the enzyme the patient lacked.
Only 1% of liver cells would have to be corrected to successfully treat the disease, said Madeux’s physician and study leader, Dr Paul Harmatz.
Safety worries plagued some earlier gene therapies. One potential problem is that the virus might provoke an immune system attack, which caused the death of 18-year-old Jesse Gelsinger during a gene therapy study in 1999. However, the new studies use a different virus that has proved much safer in other experiments.
Another worry is that inserting a new gene might have unforeseen effects on other genes. That was the case during a gene therapy trial attempting to cure a rare immune system disorder known as “bubble boy disease”. Several patients later developed leukaemia because the new gene entered a place in the native DNA where it unintentionally activated a cancer gene.
“When you stick a chunk of DNA in randomly, sometimes it works well, sometimes it does nothing and sometimes it causes harm,” said Hank Greely, a Stanford University bioethicist. “The advantage with gene editing is you can put the gene in where you want it.”
Finally, some fear that the virus could get into other places like the heart, or eggs and sperm where it could affect future generations. Doctors say built-in genetic safeguards prevent the therapy from working anywhere but the liver, like a seed that only germinates in certain conditions.
Meanwhile, Madeaux remains optimistic. “I’m nervous and excited,” he said. “I’ve been waiting for this my whole life: something that can potentially cure me.








Comments (…)
Sign in or create your Guardian account to join the discussion