Many of the polymers that make up the items we rely on are relatively easy to break down. An otherwise robust styrofoam container will dissolve completely in a solvent like chloroform. Thermoplastics like the ones found in food delivery containers get soft at higher temperatures. Raise the heat a bit more, and they'll melt. These properties help make many of the plastics we use recyclable.
But there are other plastics, called thermosets, that don't show this kind of behavior. They are extremely durable, they will shrug off solvents, and they will hold up well to heat, which makes them great for the places you've probably come across them—things like auto interiors and cases for electronic devices. The problem is that the same properties that make them so robust also make them nearly impossible to recycle.
Now, materials scientists have come up with a new thermoset polymer that's quite tough and holds up to high temperatures and most solvents. Its Achilles' heel? Strong acids. Put it in a solution with a pH below 2, and it will break back down into its component molecules, which can then be reused to form the same polymer all over again.
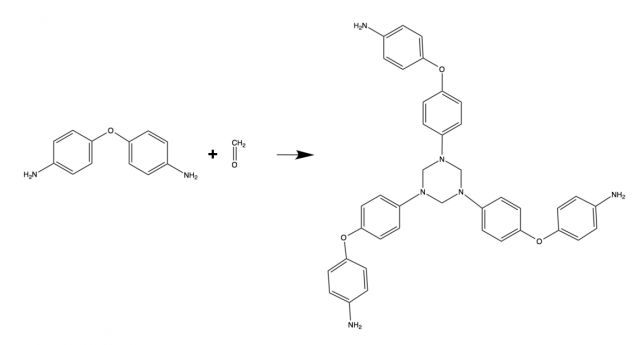
The basic building block of the polymer is the piece at left with the two rings. The key groups are the nitrogens hanging off each end. When placed in the presence of formaldehyde at 200°C, these nitrogens will react with the carbon in the formaldehyde. Each nitrogen and each carbon can react twice. The formation of a nitrogen-carbon-nitrogen combination kicks off two hydrogens from the nitrogen and an oxygen from the formaldehyde, releasing water. As the nitrogens continue to react, they create a ring, as shown above.
That's just on one side of the molecule; the nitrogen at the other side can be undergoing similar reactions, creating a polymer of linked rings. The end result is stable at high temperatures and in solvents, and it could hold up to 140,000 atmospheres of pressure. The researchers found that adding some carbon nanotubes to the reaction mix strengthened it even further.
But although the polymer was very stable, it could be broken down slowly under acidic conditions. The end product was the same molecule that the polymer started with (the one on the left in the diagram above)—after a bit of cleanup, you would end up with the raw materials for a new polymer.
The one obvious drawback to this reaction is that it requires formaldehyde, an extremely toxic chemical. But the upside of having a fully recyclable thermoset is substantial. Since such plastics are so durable, they tend to stick around in the environment for a while. And as the authors note, the difficulty of manipulating thermoset pieces once they've been manufactured means that a single, small defect can cause an entire part to be discarded.
Beyond the thermoset they demonstrate, the team behind this work also showed that the nitrogen-formaldehyde polymerization reaction works when the nitrogen is attached to a very different chemical (in the other example, they created a flexible, low-melt gel). So there's potentially a lot of additional chemistry to be explored.
Science, 2014. DOI: 10.1126/science.1251484 (About DOIs). Listing image by Bo Eide.
Listing image by Bo Eide
reader comments
21