Abstract
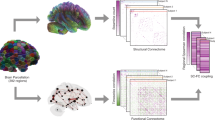
Functional magnetic resonance imaging (fMRI) studies typically collapse data from many subjects, but brain functional organization varies between individuals. Here we establish that this individual variability is both robust and reliable, using data from the Human Connectome Project to demonstrate that functional connectivity profiles act as a 'fingerprint' that can accurately identify subjects from a large group. Identification was successful across scan sessions and even between task and rest conditions, indicating that an individual's connectivity profile is intrinsic, and can be used to distinguish that individual regardless of how the brain is engaged during imaging. Characteristic connectivity patterns were distributed throughout the brain, but the frontoparietal network emerged as most distinctive. Furthermore, we show that connectivity profiles predict levels of fluid intelligence: the same networks that were most discriminating of individuals were also most predictive of cognitive behavior. Results indicate the potential to draw inferences about single subjects on the basis of functional connectivity fMRI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mangin, J.F. et al. A framework to study the cortical folding patterns. Neuroimage 23 (suppl. 1): S129–S138 (2004).
Amunts, K., Malikovic, A., Mohlberg, H., Schormann, T. & Zilles, K. Brodmann's areas 17 and 18 brought into stereotaxic space-where and how variable? Neuroimage 11, 66–84 (2000).
Bürgel, U. et al. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 29, 1092–1105 (2006).
Grabner, R.H. et al. Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage 38, 346–356 (2007).
Newman, S.D., Carpenter, P.A., Varma, S. & Just, M.A. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia 41, 1668–1682 (2003).
Rypma, B. & D'Esposito, M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc. Natl. Acad. Sci. USA 96, 6558–6563 (1999).
Mueller, S. et al. Individual variability in functional connectivity architecture of the human brain. Neuron 77, 586–595 (2013).
Van Essen, D.C. et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79 (2013).
Barch, D.M. et al. Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage 80, 169–189 (2013).
Shen, X., Tokoglu, F., Papademetris, X. & Constable, R Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage 82, 403–415 (2013).
Bianciardi, M. et al. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. Neuroimage 45, 160–168 (2009).
Jiang, T., He, Y., Zang, Y. & Weng, X. Modulation of functional connectivity during the resting state and the motor task. Hum. Brain Mapp. 22, 63–71 (2004).
Stevens, W.D., Buckner, R.L. & Schacter, D.L. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb. Cortex 20, 1997–2006 (2010).
Fischl, B. et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 14, 11–22 (2004).
Buckner, R.L., Krienen, F.M., Castellanos, A., Diaz, J.C. & Yeo, B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322–2345 (2011).
Van Dijk, K.R., Sabuncu, M.R. & Buckner, R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438 (2012).
Cattell, R.B. Intelligence: Its Structure, Growth and Action: Its Structure, Growth and Action (Elsevier, 1987).
Deary, I.J., Whalley, L.J., Lemmon, H., Crawford, J.R. & Starr, J.M. The stability of individual differences in mental ability from childhood to old age: follow-up of the 1932 Scottish Mental Survey. Intelligence 28, 49–55 (2000).
Colom, R. & Flores-Mendoza, C.E. Intelligence predicts scholastic achievement irrespective of SES factors: evidence from Brazil. Intelligence 35, 243–251 (2007).
Strenze, T. Intelligence and socioeconomic success: a meta-analytic review of longitudinal research. Intelligence 35, 401–426 (2007).
Gottfredson, L.S. Intelligence: is it the epidemiologists' elusive “fundamental cause” of social class inequalities in health? J. Pers. Soc. Psychol. 86, 174 (2004).
Chandola, T., Deary, I., Blane, D. & Batty, G. Childhood IQ in relation to obesity and weight gain in adult life: the National Child Development (1958) Study. Int. J. Obes. (Lond.) 30, 1422–1432 (2006).
Bilker, W.B. et al. Development of abbreviated nine-item forms of the Raven's Standard Progressive Matrices Test. Assessment 19, 354–369 (2012).
Cole, M.W., Bassett, D.S., Power, J.D., Braver, T.S. & Petersen, S.E. Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251 (2014).
Smith, S.M. et al. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 106, 13040–13045 (2009).
Martuzzi, R., Ramani, R., Qiu, M., Rajeevan, N. & Constable, R.T. Functional connectivity and alterations in baseline brain state in humans. Neuroimage 49, 823–834 (2010).
Laumann, T.O. et al. Functional system and areal organization of a highly sampled individual human brain. Neuron 87, 657–670 (2015).
Gabrieli, John D.E., Ghosh, Satrajit S. & Whitfield-Gabrieli, S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron 85, 11–26 (2015).
Castellanos, F.X., Di Martino, A., Craddock, R.C., Mehta, A.D. & Milham, M.P. Clinical applications of the functional connectome. Neuroimage 80, 527–540 (2013).
Kelly, C., Biswal, B.B., Craddock, R.C., Castellanos, F.X. & Milham, M.P. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn. Sci. 16, 181–188 (2012).
Zilles, K., Armstrong, E., Schleicher, A. & Kretschmann, H.J. The human pattern of gyrification in the cerebral cortex. Anat. Embryol. (Berl.) 179, 173–179 (1988).
Hill, J. et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J. Neurosci. 30, 2268–2276 (2010).
Miranda-Dominguez, O. et al. Connectotyping: model based fingerprinting of the functional connectome. PLoS ONE 9, e111048 (2014).
Cole, M.W. et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 16, 1348–1355 (2013).
Power, J.D. et al. Functional network organization of the human brain. Neuron 72, 665–678 (2011).
Cole, M.W., Yarkoni, T., Repovš, G., Anticevic, A. & Braver, T.S. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J. Neurosci. 32, 8988–8999 (2012).
Choi, Y.Y. et al. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J. Neurosci. 28, 10323–10329 (2008).
Kanai, R. & Rees, G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 12, 231–242 (2011).
Fornito, A. & Harrison, B.J. Brain connectivity and mental illness. Front. Psychiatry 3, 72 (2012).
Greicius, M. Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 21, 424–430 (2008).
Cuthbert, B.N. & Insel, T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126 (2013).
Insel, T. et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751 (2010).
Hutchison, R.M. et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378 (2013).
Hampson, M. et al. Intrinsic brain connectivity related to age in young and middle aged adults. PLoS ONE 7, e44067 (2012).
Meunier, D., Achard, S., Morcom, A. & Bullmore, E. Age-related changes in modular organization of human brain functional networks. Neuroimage 44, 715–723 (2009).
Scheinost, D. et al. Sex differences in normal age trajectories of functional brain networks. Hum. Brain Mapp. 36, 1524–1535 (2014).
Craddock, R.C., James, G.A., Holtzheimer, P.E., Hu, X.P. & Mayberg, H.S. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 33, 1914–1928 (2012).
Van Essen, D.C., Glasser, M.F., Dierker, D.L., Harwell, J. & Coalson, T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex 22, 2241–2262 (2012).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Glasser, M.F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013).
Joshi, A. et al. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics 9, 69–84 (2011).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069 (2010).
Acknowledgements
Data were provided in part by the Human Connectome Project, WU-Minn Consortium (principal investigators, D. Van Essen and K. Ugurbil; 1U54MH091657) funded by the 16 US National Institutes of Health (NIH) institutes and centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. This work was also supported by NIH grant EB009666 (R.T.C.), T32 DA022975 (D.S.) and the US National Science Foundation Graduate Research Fellowship Program (E.S.F. and M.D.R.).
Author information
Authors and Affiliations
Contributions
E.S.F., X.S., D.S., X.P. and R.T.C. conceptualized the study. X.S. designed and performed the identification analyses with support from E.S.F. and D.S. E.S.F. designed and performed the behavioral analyses with support from M.D.R. and J.H. X.S. and X.P. contributed unpublished data analysis tools and visualization software. X.P., M.M.C. and R.T.C. provided support and guidance with data interpretation. E.S.F. wrote the manuscript, with contributions from X.S. and comments from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3 (PDF 289 kb)
Rights and permissions
About this article
Cite this article
Finn, E., Shen, X., Scheinost, D. et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 18, 1664–1671 (2015). https://doi.org/10.1038/nn.4135
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4135
This article is cited by
-
Longitudinal changes in resting state fMRI brain self-similarity of asymptomatic high school American football athletes
Scientific Reports (2024)
-
Brain-based graph-theoretical predictive modeling to map the trajectory of anhedonia, impulsivity, and hypomania from the human functional connectome
Neuropsychopharmacology (2024)
-
Individual differences in the neural architecture in semantic processing
Scientific Reports (2024)
-
Targeting suicidal ideation in major depressive disorder with MRI-navigated Stanford accelerated intelligent neuromodulation therapy
Translational Psychiatry (2024)
-
FAformer: parallel Fourier-attention architectures benefits EEG-based affective computing with enhanced spatial information
Neural Computing and Applications (2024)