Abstract
The upconversion luminescence (UCL) enhancement based on the surface plasmonic resonance (SPR) of noble metals is a promising way to improve UCL efficiency. However, it is still a challenge to achieve stable and effective UCL enhancement. Here, we present the preparation of the porous Ag/YVO4:Yb3+, Er3+ composite film via a simple double annealing method. It is exciting to observe that a maximum 36-fold (2H11/2–4I15/2) and 30-fold (4S3/2–4I15/2) UCL enhancement in the porous Ag/YVO4:Yb3+, Er3+ composite film, attributed to the effective coupling between SPR and the excitation light by adjusting the SPR peak to the excitation wavelength, controlling the effective coupling distance and improving the scattering–absorption ratio. Furthermore, the enhancement factor strongly depended on the excitation power and the Er3+ concentration.
Export citation and abstract BibTeX RIS
1. Introduction
Rare-earth (such as Yb3+, Er3+/Tm3+) doped upconversion luminescence (UCL) nanocrystals (NCs), which can emit ultraviolet–visible (UV–vis) light as well as absorb near infrared (NIR) light successively, has attracted extensive interest in the field of solar cell, biological fluorescence imaging, detections and fingerprint etc [1–4]. Because upconversion nanocrystals (UCNCs) have several advantages compared to the conventional photoluminescence material such as dyes and quantum dots, including the upconversion characters, low toxicity, high chemical stability and a high-signal-noise-ratio [5–9]. Unfortunately, the lower UCL efficiency of UCNCs and the smaller absorption cross-section of Yb3+ ions limit their practical applications [10]. Up to now, many works have been adopted to improve UCL signals, such as the suitable host selection [11], core–shell structure design [12], photonic crystals effect [13, 14], metal induced enhancement etc [15, 16]. In various methods, surface plasmon resonance (SPR) of noble metal is a promising way to improve UCL efficiency, which is the collective electron oscillation on a metal surface. The essence of SPR induced UCL enhancement can be mainly divided into two parts: (1) the excitation field enhancement; (2) the increase of the intrinsic radiative transition rates. So the guiding principle for the UCL enhancement is to tune the SPR peak to the excitation/emission wavelength of the luminescence NCs [17–20].
Furthermore, in order to obtain the high efficiency UCL in metal/emitters coupling system, the other two factors should be considered. One is the controllability of the effective coupling distance between metal and emitters. In general, the SPR enhancement is a near-field effect and decays nearly exponentially away from the metal surface [21, 22]. The other one is the improvement of the scattering–absorption cross-section ratio of metal nanoparticles (NPs). Because the nonradiative and radiative rates of emitters in the metal/nanophosphors system are essentially determined to their absorption- and scattering-cross-sections of metal NPs at the emission wavelength. The ratio between scattering and absorption depends on the size of metal NPs, and the bigger size of metal NPs has the higher scattering–absorption cross-section ratio [23–25]. However, the previous works were mainly focused on the hybrids in the colloids (such as the YVO4/NaYF4:Yb3+, Er3+ @Ag(Au) core–shell design) [26–29]. In metal/UCL core–shell hybrids, on one hand, the reaction temperature, surfacts and solutions in the preparation process would largely affect the UCL of emitters, which is not conducive to find out the essence reason of UCL enhancement, as reported in the previous work [30]. On the other hand, it is difficult to tune the SPR peak to the excitation/emission wavelength as well as control the effective coupling distance, leading to the low coupling efficiency between metal and emitters [31].
In our work, the hybrids consisting of a porous Ag film (Ag NPs > 100 nm) and the small size of YVO4:Yb3+, Er3+ UCNCs prepared by sol–gel method were prepared through a double annealing method. In this paper, we observed that the porous Ag film has super-broad SPR peak ranging of 350–1100 nm, covering the excitation/emission wavelength of emitters. It should be highlighted that a maximum 36-fold (2H11/2–4I15/2) and 30-fold (4S3/2–4I15/2) UCL enhancement was realized in the Ag/YVO4:Yb3+, Er3+ composite film, which is higher than that reported in the previous literatures [32, 33].
2. Experimental methods
2.1. Sample preparation
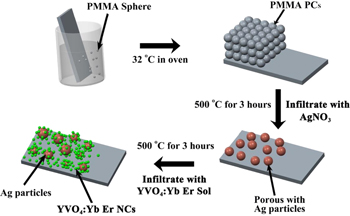
The porous Ag film was prepared by the PMMA opal photonic crystals (OPCs) template removal method, and the OPCs were prepared according to our previous works [34]. The specific experiments are as follows. First, the OPCs were penetrated with a solution of AgNO3 in a mixture of water and ethanol. After infiltration, the product was dried in air at room temperature for 30 min. The annealing was carried out by slowly increasing the temperature (50 °C h−1) up to 500 °C for 3 h, then a porous Ag film formed. In the preparation process of the YVO4: (15 mol%)Yb3+, xEr3+ (x = 1, 2, 3, 5, 8 mol%) precursor sol, the stoichiometric amounts of Y(NO3)3 · 6H2O, Yb(NO3)3 · 6H2O, Er(NO3)3 · 6H2O and NH3VO4 was first dissolved in ethanol solution. The ethanol solution contained citric acid as the chelating agent. Then, appropriate amount of nitric acid was dissolved in ethanol. The mixture was stirred for 4 h, forming a transparent solution. The mixture solution was infiltrated in the voids of the porous Ag film. The further annealing was carried out with slowly elevated temperature (40 °C h−1) up to 500 °C for 3 h. Finally, the porous Ag/YVO4:Yb3+, Er3+ NCs hybrids were formed. The preparation process is shown in scheme
Scheme 1. Schematic illustration of the porous Ag/YVO4:Yb3+, Er3+ composite film formation process.
Download figure:
Standard image High-resolution image2.2. Characterization and measurements
The samples were characterized with a JEOL JSM-7500 field emission scanning electron microscope at an accelerating voltage of 15 kV, and a Hitachi H-800 transmission electron microscope (TEM) operated at an acceleration voltage of 200 Kv. The crystalline structure of the samples was characterized by x-ray diffraction (XRD) (RigakuD/max-rA power diffractometer using Cu–KR radiation (λ)1.54178 Å). (UV–vis) absorption spectra were measured with a Shimadzu UV-3101PC UV–vis scanning spectrophotometer ranging of 200–1100 nm. A continuous 980 nm diode laser was used to pump the samples when investigating the steady-state spectra. The luminescent dynamics of Er3+ ions were investigated by a laser-system consisting of a Nd:YAG pumping laser (1064 nm), the third-order Harmonic-Generator (355 nm) and a tunable optical parameter oscillator (OPO, Continuum Precision II 8000). The laser has pulse duration of 10 ns, repetition frequency of 10 Hz and line width of 4–7 cm−1.
3. Results and discussion
3.1. Morphology and structure of the porous Ag/YVO4:Yb3+, Er3+ hybrids
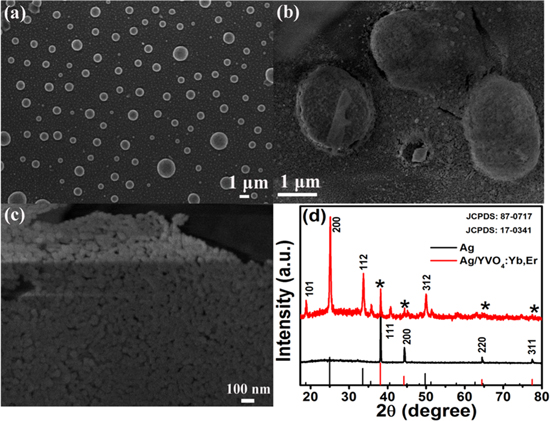
Figure 1(a) shows the SEM image of the porous Ag film, which indicates that the Ag film is composed of randomly distributed and regular Ag spheres, ranging in size from 100 to 2000 nm, with many pores among the Ag spheres formed during the annealing. Figure 1(b) records the SEM image of the porous Ag/YVO4:Yb3+, Er3+ film. It can be seen that the YVO4:Yb3+, Er3+ UCNCs were mainly filled in the pores between the Ag particles and covered on the surface of the Ag NPs, indicating the formation of Ag/YVO4:Yb3+, Er3+ hybrids. As a comparison, the SEM image of YVO4:Yb3+, Er3+ film on the glass substrate (as the reference sample) was shown in figure 1(c), indicating that very thin but continuous YVO4:Yb3+, Er3+ films were formed on the glass substrate, consisting of closely packed UCNCs, with the size of YVO4:Yb3+, Er3+ NCs being ∼15 nm. Figure 1(d) represents the XRD patterns of the porous Ag film and the porous Ag/YVO4:Yb3+, Er3+ composite film on the glass substrate and the corresponding standard cards, JCPDS 87–0717 for cubic silver and 17–0341 for tetragonal phase YVO4. In the Ag/ YVO4:Yb3+, Er3+ composite film, the (111) face of cubic silver and the characteristic diffraction peaks at (200), (112) and (312) of tetragonal phase YVO4 can be clearly distinguished, and no impurity peaks appeared, indicating the formation of the porous Ag/YVO4:Yb3+, Er3+ composite film. In most of the previous works, the metal/luminescence centers hybrids were prepared according to the chemical control methods, which introduced some species, such as surfactants and solvents, would significantly affect the effective of UCL and the stable of noble metal NPs [35, 36]. As a comparison, in the porous Ag film/YVO4:Yb3+, Er3+ hybrids prepared by the PMMA template removal method not only can rule out the effect of the large phonon groups on the UCL, but also improving the stability of the Ag particles. Furthermore, the SPR peak of metal (the excitation wavelength) and the coupling distance between UCNCs and metal NPs is easy to control in our system, which leads to stable and reproducible UCL enhancement.
Figure 1. SEM images of the porous Ag film (a), the porous Ag/YVO4:Yb3+, Er3+ composite film (b), the reference YVO4:Yb3+, Er3+ on the glass (c). (d) XRD patterns of the porous Ag film and as-prepared Ag/YVO4:Yb3+, Er3+ composite film with the standard cards for YVO4 and cubic silver for comparison.
Download figure:
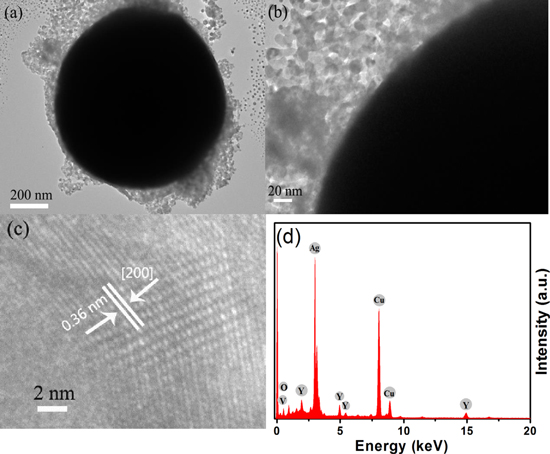
Standard image High-resolution imageIn order to further determine the structure of the porous Ag/YVO4:Yb3+, Er3+ hybrid film, the hybrid was scraped from the glass substrate and dispersed in ethanol, then dropped onto a copper grid for high-resolution TEM (HR-TEM) measurements. Figures 2(a) and (b) respectively show low and high magnification TEM images of Ag/YVO4:Yb3+, Er3+ hybrids. It can be clearly seen that the YVO4:Yb3+, Er3+ UCNCs were adsorbed on the surface of the Ag particle and the thickness of the surface YVO4:Yb3+, Er3+ layer on the Ag particle was in the range of 20–100 nm. The obvious lattice fringes confirm the high crystallization of the samples, as shown in the HR-TEM image (figure 2(c)). A distance of about 0.36 nm between adjacent lattice fringes could be well assigned as the d spacing value of the (200) plane of YVO4. The energy-dispersive x-ray (EDX) analysis in panel d of figure 2(d) shows the presence of Y V and O with an approximate stoichiometry of YVO4, furthermore, the spectrum confirms the presence of Ag in the sample. Note that the peaks of C and Cu come from the surface of the copper grid used for TEM measurement. The EDX results indicate that the Ag/YVO4:Yb3+, Er3+ hybrids were indeed formed.
Figure 2. (a) Low and (b) high magnification TEM images of porous Ag/YVO4:Yb3+, Er3+ hybrids. (c) Typical HR-TEM image of YVO4:Yb3+, Er3+ on the surface of an Ag particle. (d) EDX spectra of as-prepared Ag/YVO4:Yb3+, Er3+ hybrids.
Download figure:
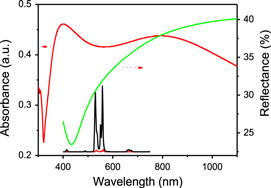
Standard image High-resolution imageThe absorption and reflectance spectra of the Ag nano-film were measured, as shown in figure 3. It's interesting to observe that the absorption of the porous Ag film is a broad band extending from 350 to 1100 nm, which largely differs from the absorption of Ag NPs and can be attributed to the strong plasmon coupling among the neighboring Ag NPs. From the reflection spectrum of the Ag nano-film, the reflectance is found to be about 30% in the range of 520–550 nm, and it gradually increases with increasing wavelength. In the NIR region (980 nm), the reflectance approaches about 40%. The UCL spectra in YVO4:Yb3+, Er3+ samples were also recorded under the excitation of a 980 nm laser diode in air, as shown in figure 3. The UCL of Er3+ ions was clearly observed, mainly determined to green (2H11/2, 4S3/2–4I15/2) and red (4F9/2–4I15/2) transitions, respectively. In contrast to YVO4:Yb3+, Er3+ film, the overall fluorescence intensity of Er3+ in the Ag/YVO4: Yb3+, Er3+ composite film sample increases up to 36 and 30 fold for 2H11/2–4I15/2 and 4S3/2–4I15/2 transitions. It should be noted that the SPR absorption of the porous Ag film covers the entire excitation (∼980 nm)/emission region (400–700 nm) of YVO4: Yb3+, Er3+ samples.
Figure 3. Absorption and reflectance spectra of the Ag nano-film, and a comparison of emission spectra between YVO4:Yb3+, Er3+ and Ag/YVO4:Yb3+, Er3+ composite film samples.
Download figure:
Standard image High-resolution image3.2. More effective UCL enhancement of porous Ag/YVO4:Yb3+, Er3+ hybrids
In order to investigate the modulation on the UCL of YVO4: Yb3+, Er3+ UCNCs based on the porous Ag film. Figure 4(a) records the enhancement factor (EF) versus the 980 nm excitation power (EF is defined as the ratio of integral intensity between the porous Ag/YVO4: Yb3+, Er3+ film and YVO4: Yb3+, Er3+ film). It can be seen that the EF depends strongly on excitation power as well as the emission wavelength. It's exciting to observe that the maximum EF of the 2H11/2–4I15/2 and 4S3/2–4I15/2 transitions are about 36 fold and 30 fold, respectively, corresponding to a low excitation power of 120 mW. Based on our previous work [33], the EF in porous Ag film is obviously higher than in dense Ag film, because the UCNCs is convenient to fill in the porous Ag film and on the surface of Ag particles, which is useful for decreasing the coupling distance between UCNCs and Ag NPs, while in the dense Ag film, the UCNCs can only locate at the top of the Ag film layer. On the other hand, the UCL enhancement has been influenced by the scattering–absorption ratio, it is usually expected that the absorption term to cause quenching and the scattering term to cause enhancement. The absorption term increases as r3 (r is the radius of Ag sphere) where the scattering term increases as r6 [19]. So the large size (100–2000 nm) of Ag NPs is benefit for the UCL enhancement. And as the excitation power increases, the EF of all the transitions gradually decreases, which should be due to the local thermal effect induced by the laser exposure. In order to better understand the fact above, the UC emission intensity as a function of excitation power for the 2H11/2, 4S3/2–2H15/2 transitions were recorded, as shown in figures 4(b) and (c). It can be seen that the values of slope n (IUCLIn) in Ag/YVO4: Yb3+, Er3+ (1.03 for 2H11/2–4I15/2, 0.54 for 4S3/2–4I15/2) are smaller than those in YVO4: Yb3+, Er3+ (1.76 for 2H11/2–4I15/2, 1.62 for 4S3/2–4I15/2), and the slope n for the emissions of Er3+ ions are all smaller than the required photon number (n = 2) for generating the corresponding transitions, even at low excitation power. All the facts above can be attributed to the saturation effect as well as the local thermal effect induced by the laser exposure [37]. Figure 4(d) displays the deduced local temperature (based on RHS) of the sample as a function of 980 nm excitation power. It can be seen that the porous Ag/YVO4: Yb3+, Er3+ composite film has a relatively higher temperature than the YVO4: Yb3+, Er3+ nano-film, which is due to the absorption by the silver nano-film of the 980 nm excitation light and the further photo-thermal transfer, leading to the increase of nonradiative relaxation of Er3+ ions and the decrease of EF. This result is different to our previous reports, which would be due to the different Ag density and the different preparation process [32, 33]. It can be concluded that in the porous Ag/YVO4: Yb3+, Er3+ composite film, the coupling distance between Ag particles and YVO4:Yb3+, Er3+ NCs have been effectively reduced. It is well known that the maximum electric field intensity enhancement occurs mostly in the regions close to the surface of Ag NPs (within a few nanometers). So the EF has been improved considerably. Then on the other hand, as to the high Ag density and Yb3+ doped concentration in YVO4 (the phonon energy ∼870 cm−1), all of these can absorb 980 nm excitation light and effectively photo-thermal transfer, and the local thermal effect in Ag/YVO4:Yb3+, Er3+ will become serous relative to the YVO4:Yb3+, Er3+ thin film sample at the high 980 nm excitation power, so the EF decrease with the increase of the excitation power.
Figure 4. The power-dependent enhancement factor (a), the upconversion emission intensity for the 2H11/2, 4S3/2–2H15/2 transitions as ln–ln plots (b),(c) and the deduced sample temperature (d) as a function of excitation power of the YVO4:Yb3+, Er3+ nano-film and the Ag/YVO4:Yb3+, Er3+ composite film.
Download figure:
Standard image High-resolution image3.3. Dependence of UCL enhancement on Er3+ doped concentration
In order to better understand the origin of UCL enhancement, the concentration-dependent UCL EF behavior in porous Ag/YVO4:Yb3+, Er3+ films, the UCL dynamic processes of the excited states 4S3/2 for different doped concentration of Er3+ ions were investigated. Figure 5(a) records the decay process of the 4S3/2–4I15/2 transitions of Er3+ ions (1 mol%) under the excitation of a 980 nm pulsed laser, which can be well fitted to the single exponential function. The decay time constants for Er3+ ions in YVO4:Yb3+, Er3+ and Ag/YVO4:Yb3+, Er3+ film samples are 11 μs and 9 μs, respectively. The reduction (∼20%) of decay time constant in Ag/YVO4:Yb3+, Er3+ composite film can be mainly attributed to the nonradiative relaxation induced by the metal thermal effect. Considering that the porous Ag film had a broad SPR ranging from 320 nm to 1100 nm overlapping with all the emissions of YVO4:Yb3+, Er3+ in this range (including 2F5/2–2F7/2 of Yb3+ at 980 nm) and the decay time constants of the green emission (4S3/2–4I15/2) for Er3+ ions are nearly unchanged. This indicates that the remarkable UCL enhancement was mainly induced by the coupling of the 980 nm excitation light with the SPR, rather than an increase in radiative transition rates of Er3+/Yb3+ ions due to the coupling of surface plasmon absorption of silver with emitted light. Combined the discussions above, the remarkable UCL enhancement originates from the effective coupling between SPR and the excitation light by adjusting the SPR peak to the excitation wavelength, controlling the effective coupling distance and improving the scattering–absorption ratio.
Figure 5. (a) UCL decay curves of the transition 4S3/2–4I15/2 (λem = 556 nm) under the 980 nm excitation. (b),(c) Inverse of 4S3/2–4I15/2 decay time constants and EF versus concentration of Er3+ ions in YVO4: (15 mol%)Yb3+, Er3+ and Ag/YVO4 (15 mol%)Yb3+, Er3+ film samples. (d) The corresponding energy-level diagram of Yb3+/Er3+ co-doped YVO4 host.
Download figure:
Standard image High-resolution imageFigure 5(b) shows the EF of the 2H11/2, 4S3/2–4I15/2 transitions for Er3+ on doping concentration of Er3+ in different sample series, in YVO4:Yb3+, Er3+ and Ag/YVO4:Yb3+, Er3+ film samples. It can be seen that as the concentration increased from 1 mol% to 8 mol%, EF gradually decreased from ∼36-fold to 17-fold for 2H11/2–4I15/2 transition and ∼30-fold to 14-fold for 4S3/2–4I15/2 transition compared to the YVO4:Yb3+, Er3+ film. EF was proportional to the inverse of Er3+ ions concentration, which is due to the increase of the cross relaxation processes among Er3+ ions with the increasing of Er3+ ions concentration. To clarify the origin of the decrease of EF with increasing of Er3+ ions concentration, the dependence of exponential spontaneous decay rates (SDR) of 4S3/2–4I15/2 for Er3+ on doping concentration of Er3+ in different sample series were presented in figure 5(c). It can be seen that in the Ag/YVO4:Yb3+, Er3+ film samples the SDR of 4S3/2–4I15/2 increases rapidly, while in the YVO4:Yb3+, Er3+ the SDR of 4S3/2–4I15/2 increases slowly with the increasing Er3+ concentration. Based on previous literatures, the SDR of 4S3/2–4I15/2 transition can be written as,
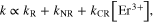
where, k, kR and kNR represent the total SDR, the radiative decay rate and the nonradiative relaxation rate of 4S3/2, respectively, and kCR represents the cross relaxation rate from 4S3/2. Based on the above equation, the values of (kR + kNR) in YVO4:Yb3+, Er3+ and Ag/YVO4:Yb3+, Er3+ film samples were deduced to be 75.5 ms−1 and 64.4 ms−1, respectively, while the values of kCR in YVO4:Yb3+, Er3+ and Ag/YVO4:Yb3+, Er3+ film samples were deduced to be 13.6 ms−1 mol−1 and 27.8 ms−1 mol−1. In Ag/YVO4:Yb3+, Er3+ samples, the local temperature is higher than that in YVO4:Yb3+, Er3+ samples due to the absorption by the silver nano-film of the 980 nm excitation light and effective photo-thermal transfer, the cross relaxation processes happened largely in the high temperature. So the EF decreases with the increasing in the concentration of Er3+ ions. The simple schematic of UC populating and emission processes in Er3+, Yb3+-doped YVO4 was drawn in figure 5(d).
To clarify the origin of luminescent enhancement, first, let us consider the influence of the porous Ag film on the radiative and nonradiative transition rates of Er3+ ions. It is apparent that the radiative decay rate in the Ag/YVO4:Yb3+, Er3+ film sample almost unchanged relative to YVO4:Yb3+, Er3+ film sample, while the value of nonradiative relaxation rate increases about 2 times. So the decrease of decay time constants (20%) could be attributed to the increase of the nonradiative rate, instead of the increase of the radiative rate induced by the emission coupling. Second, let us consider the optical reflection effect of the Ag film on the UCL. The transmittance of the YVO4:Yb3+, Er3+ nano-film to the 980 nm light is 68% (measured by absorption spectroscopy), and the reflection of the Ag film to the 980 nm light is 40% and to the visible emissions is about 30%. Thus it can be roughly estimated that due to the reflection of the Ag film, the excitation light increases about 27%, while the emission light increases about 30%. Totally, the visible UCL should increase up to 1.57 times due to the reflection of the Ag film. This fact indicates that the UCL enhancement can be mainly attributed to the excitation filed enhancement induced by the coupling of SPR with the excitation light, instead of the emission coupling.
4. Conclusion
In this work, the Ag/ YVO4:Yb3+, Er3+ composite film samples were prepared via a double annealing method. It is with strong SPR of silver, ranging from 320 to 1100 nm. Because of the strong coupling between the 980 nm excitation light and SPR of Ag NPs, the remarkable UCL enhancement (36-fold for 2H11/2–4I15/2 transition and 30-fold for 4S3/2–4I15/2 transition) emissions in the low excitation power were observed. The EF depended strongly on the excitation power and Er3+ ions concentration. In metal/UCNCs system, this work provides a simple way to realize the highly efficient, stable and repeatable UCL enhancement through controlling the coupling between SPR and excitation light, effective distance and scattering–absorption ratio.
Acknowledgments
This work was supported by the Major State Basic Research Development Program of China (973 Program) (No. 2014CB643506), the National Natural Science Foundation of China (Grant Nos. 61204015, 51002062, 11174111, 61177042 and 81201738), and Program for Chang Jiang Scholars and Innovative Research Team in University (No. IRT13018).